Introduction
Understanding the specific types of strain is crucial for fully grasping cycloalkane stability. The total Ring Strain is the sum of three main components:
Ring Strain = Angle Strain + Torsional Strain + Steric Strain
Angle Strain (EA)
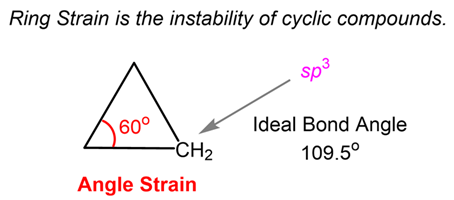
- Definition: Strain caused by the deviation of the C-C-C bond angles (α)from the ideal tetrahedral angle of 109.50.
- Cause: Forcing the sp3 hybridized carbon orbitals to form bonds at angles different from their natural 109.50.
- Effect on Stability: Increases the potential energy of the molecule.
- Relevance:
- High in Cyclopropane (α=600) and Cyclobutane (α≈880) in the puckered form).
- Low in Cyclopentane (α≈1050) in the envelope form or half-chair conformation, where one carbon atom is out of the plane of the other four.
- Zero in the Chair Conformation of Cyclohexane (all α =109.50).
Torsional Strain (ET)
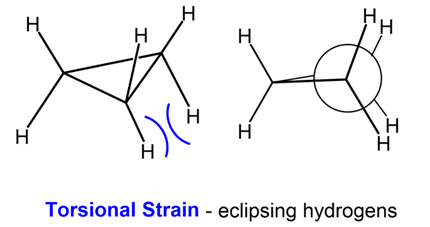
- Definition: Strain caused by the eclipsing of C-H bonds on adjacent carbon atoms. This is also known as eclipsing strain.
- Cause: When the dihedral angle (θ) between the bonds on adjacent carbons is 00 (eclipsed conformation), the electron clouds of the bonds repel each other, raising the energy. Stability is maximized when the bonds are staggered (θ = 600).
- Effect on Stability: Raises energy; leads to hindered rotation in acyclic alkanes.
- Relevance:
- High in Cyclopropane and Cyclobutane because the rigid, near-planar structure forces many C-H bonds into an eclipsed or nearly eclipsed arrangement.
- Still present in the slightly puckered Cyclopentane (in its envelope or half-chair form).
- Zero in the highly stable Chair Conformation of Cyclohexane (all adjacent C-H bonds are perfectly staggered).
- Present in the Boat Conformation of Cyclohexane (eclipsed C-H bonds on C2-C3 and C5-C6).
Steric Strain (ES)
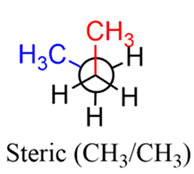
- Definition: Strain caused by non-bonded repulsion when two atoms or groups are forced to occupy the same space or come closer than their van der Waals radii allow. This is often called Van der Waals Strain.
- Cause: Large groups trying to occupy close spatial proximity, leading to repulsive forces between their electron clouds.
- Effect on Stability: Significant destabilization, especially in substituted or larger rings.
- Relevance (Intra-ring H-H):
- Zero in Cyclopropane and Cyclobutane (too small for internal H-H crowding).
- Present in the Boat Conformation of Cyclohexane due to the repulsion between the two H atoms pointing inwards from C1 and C4. These are called flagpole hydrogens.
- Major factor in the instability of many medium-sized rings (e.g., C7 to C13), which must twist into shapes that bring distant groups too close.
Summary of Strains in Small Rings
The total strain energy (measured via Heat of Combustion per CH2 group) confirms the combined effect of these strains:
| Cycloalkane | Bond Angle (α) | Angle Strain (EA) | Torsional Strain (ET) | Total Strain (E) kJ/mol |
| Cyclopropane | 600 | Very High | Very High (Full Eclipsing) | 115 |
| Cyclobutane | ≈ 880 | High | High (Partial Eclipsing) | 110 |
| Cyclopentane | ≈ 1050 | Low | Low (Partial Puckering) | 27 |
| Cyclohexane (Chair) | 109.50 | Zero | Zero (Full Staggering) | 0 |