Introduction
Fats and oils (triglycerides) are the primary components of many biological and industrial processes. Hydrolysis is the reverse of the esterification process that originally formed the fat. It is the chemical breakdown of a triglyceride (fat or oil) into its building blocks: glycerol and three fatty acids through the addition of water. This reaction involves the cleavage of the ester bonds that hold the fat molecule together.
Since fats and oils are not soluble in water, the reaction usually requires a catalyst (acid, base, or enzyme) and superheated steam to proceed effectively.
The Chemical Reaction
A triglyceride consists of one glycerol molecule (a 3-carbon alcohol) linked to three fatty acid chains. During hydrolysis, three molecules of water (H2O) are used to break these links in the presence of catalyst.
The Overall Reaction:
Triglyceride + 3 H2O Glycerol + 3 Fatty Acids
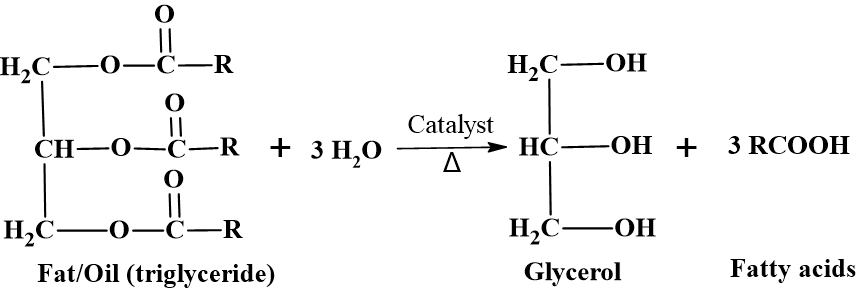
The Step-by-Step Mechanism
The reaction does not usually happen all at once. It occurs in three successive steps, where one fatty acid chain is removed at a time:
- Triglyceride → Diglyceride + 1 Fatty Acid
- Diglyceride → Monoglyceride + 1 Fatty Acid
- Monoglyceride → Glycerol + 1 Fatty Acid
Types of Hydrolysis
Since fats are hydrophobic (water-fearing) and water is hydrophilic, they do not mix well. Therefore, the reaction is very slow unless a catalyst is used. Hydrolysis can occur through several pathways depending on the environment and the desired end product.
Acidic Hydrolysis
- In the presence of a mineral acid (like HCl or H2SO4), fats are split into glycerol and free fatty acids. The acid acts as a catalyst to speed up the breaking of ester bonds.
- Free fatty acids are obtained. This is often used in laboratories to analyze the fatty acid composition of a sample.
Alkaline Hydrolysis (Saponification)
When a fat is reacted with a strong base (alkali) like Sodium Hydroxide (NaOH) or Potassium Hydroxide (KOH), the process is specifically called Saponification. The base acts as a catalyst to speed up the reaction. Instead of free fatty acids, fatty acid salts are obtained, which is known as soap.
Reaction: Triglyceride + 3NaOH → Glycerol + 3RCOONa (Soap)
Enzymatic Hydrolysis
In living organisms, fat hydrolysis is the essential first step of lipid metabolism. In the human body, the lipase enzyme in the small intestine hydrolyzes dietary fats so they can be absorbed into the bloodstream. Digestion begins in the stomach (gastric lipase) but mostly occurs in the small intestine, where pancreatic lipase breaks down dietary fats into monoglycerides and fatty acids. Because oil and water don’t mix, bile salts from the liver emulsify the large fat globules into tiny droplets. This increases the surface area for the enzymes. Now the fat molecules are small enough to pass through the intestinal wall into the bloodstream. This is how our body processes fats at a low temperature (37°C).
Steam Hydrolysis (Industrial)
Large-scale industrial production of fatty acids often uses superheated steam (2500C and high pressure) without a chemical catalyst. This is more efficient for producing pure fatty acids and glycerol for cosmetics and pharmaceuticals.
Importance and Applications
- Soap Industry: Direct alkaline hydrolysis (Saponification) is the foundation of all soap manufacturing.
- Metabolism: Hydrolysis is the first step in mobilizing stored body fat for energy. When you “burn fat,” your body first hydrolyzes triglycerides stored in adipose tissue.
- Food Spoilage (Hydrolytic Rancidity): This is a form of food spoilage. When butter or oils are left in moisture or warm environments, natural bacteria or moisture can trigger partial hydrolysis. This releases short-chain fatty acids like butyric acid, which is responsible for the “sour” or “vomit-like (foul)” smell of rancid butter.
The analytical tests used to measure the extent of hydrolysis are the Acid Value and Saponification Value.