Introduction
Hydrogenation is the chemical process of adding hydrogen atoms to the double bonds of unsaturated fatty acids in liquid oils. This reaction transforms them into more saturated, solid, or semi-solid fats.
In industry, this is often called the “Hardening of Oils”. It raises the melting point, turning a liquid (like soybean oil, canola or sunflower oil) into a semi-solid fat (like margarine or vegetable ghee).
Example: The production of Vanaspati Ghee (vegetable ghee) from liquid vegetable oils.
The Chemical Reaction
Vegetable oils are composed of triglycerides containing unsaturated fatty acids (which have one or more C=C double bonds). Hydrogenation breaks these double bonds and replaces them with single bonds by adding two hydrogen atoms per double bond.
The Reaction:
Unsaturated Oil (Liquid) + H2 Saturated Fat (Solid)
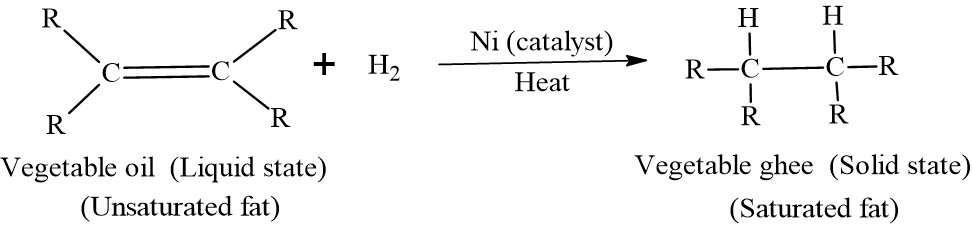
The Industrial Process (Conditions)
For the reaction to occur efficiently on a large scale, four components are required:
- Catalyst: Finely divided Nickel (Ni) is the most common industrial catalyst. It provides a surface where the hydrogen gas and the oil can meet and react.
- Temperature: Usually between 150
and 200
.
- Pressure: The reaction is carried out under high pressure of hydrogen gas to force the gas into the liquid oil.
- Agitation: The mixture is stirred vigorously to ensure the nickel catalyst remains suspended and comes into contact with both the oil and the gas.
Partial vs. Complete Hydrogenation
Manufacturers rarely hydrogenate an oil completely because the resulting fat would be as hard as a candle (brittle and waxy). Instead, they use Partial Hydrogenation.
| Type | Process | Resulting Texture | Health Impact |
| Complete | All double bonds are saturated. | Very hard, waxy solid. | High in saturated fat; no trans-fat. |
| Partial | Only some double bonds are saturated. | Soft, spreadable (Margarine). | Contains Trans-fat (side-reaction). |
The “Trans-Fat” Side Effect
During partial hydrogenation, the heat and catalyst can cause some remaining double bonds to “flip” their geometry from the natural cis form (bent) to the trans form (straight). Unlike natural fats, trans-fats are difficult for the body to process and are linked to increased heart disease risk.
Why Hydrogenate? (Benefits & Applications)
- Texture Control: It allows liquid oils to be used in baking (shortening) and spreads (margarine), providing a creamy “mouthfeel.”
- Oxidative Stability: Double bonds are the “weak points” where oxygen attacks (causing rancidity). By removing them, the oil becomes much more stable and has a significantly longer shelf life.
- High Smoke Point: Hydrogenated fats are better for deep-frying because they don’t break down as easily at high temperatures compared to liquid oils.
- Economic Value: It allows cheap vegetable oils (like soy or palm) to be converted into functional alternatives for expensive animal fats like butter or lard.
Summary of Physical & Chemical Changes
When you look at the “before and after” of hydrogenation, these four characteristics always change:
- Melting Point: Increases. This is the primary goal—turning oil into a spreadable solid.
- Oxidative Stability: Increases. Double bonds are the sites where oxygen attacks to cause rancidity. Fewer double bonds mean the fat stays fresh for much longer.
- Iodine Value: Decreases. Since the Iodine Value measures the degree of unsaturation (number of double bonds), adding hydrogen directly lowers this number.
- Refractive Index: Decreases. As the degree of saturation increases, the way the fat bends light changes.