Preparation and standardization are fundamental techniques in analytical chemistry. It is crucial to understand the principle, chemical reactions, equivalent weight calculations, and the indicators used.
Equivalent weight calculation: Eq. Wt. = Molecular Weight/Valency Factor (n-factor); The n-factor varies depending on whether the substance is an acid, a base, or a redox agent.
Calculation of the exact normality or molarity of a solution based on titration data.
The most common formula used is the Normality Equation: N1V1 = N2V2
Where:
- N1, V1: Normality and Volume of the Standard Solution (Known).
- N2, V2: Normality and Volume of the Test Solution (Unknown).
Oxalic Acid (H2C2O4 ⋅ 2H2O)
Oxalic acid is a primary standard because it is available in high purity, is stable, and has a high equivalent weight. It is a diprotic acid, meaning it releases two H+ ions per molecule.
- Preparation (0.1 N):
- Molecular Weight = (2 x 1) + (2 x 12) + (4 x 16) + (2 x 18) = 126.07 g/mol.
- Equivalent Weight = Molecular Weight/2 = 63.03.
- For 0.1 N solution, dissolve 6.303 g of oxalic acid dihydrate in distilled water and make up the volume to 1000 mL in a volumetric flask.
- Standardization: Since it is a primary standard, it does not strictly require standardization, but it is often used to standardize bases like NaOH.
Sodium Hydroxide (NaOH)
NaOH is a secondary standard because it is hygroscopic (absorbs moisture) and reacts with atmospheric CO2. It is a monoacidic base and releases one OH– ion.
- Preparation (0.1 N):
- Molecular Weight = 23 + 16 + 1= 40.0 g/mol.
- Equivalent Weight = Molecular Weight/1 = 40.0.
- For 0.1 N solution, Dissolve approximately 4.0 g of NaOH pellets in CO₂-free distilled water and make up the volume to 1000 mL in a volumetric flask.
- Standardization (using Oxalic Acid):
- Principle: Acid-base neutralization titration.
- Indicator: Phenolphthalein (Colorless to permanent pale pink).
- Reaction: H2C2O4 + 2NaOH → Na2C2O4 + 2H2O.
- Calculation: N1V1 (Acid) = N2V2 (Base).
Hydrochloric Acid (HCl)
HCl is a secondary standard as it is a volatile liquid and its exact concentration in concentrated form varies. Concentrated HCl is usually 37% w/w and ~11.6 N.
- Preparation (0.1 N):
- Strength = 37% w/w and ~11.6 N.
- Volume required = V1 = N2V2 / N1 = 0.1 × 1000 / 11.6 ≈ 8.6 mL
- For 0.1 N solution, dilute approx. 8.6 mL of concentrated HCl in distilled water and make up the volume to 1000 mL in a volumetric flask. (Note: Always add acid to water, never water to acid., to prevent vigorous splashing due to the exothermic reaction.)
- Standardization (using Sodium Carbonate):
- Primary Standard: Anhydrous Na2CO3 (dried at 270°C).
- Indicator: Methyl Orange (yellow to faint pink) or Methyl Red.
- Reaction: Na2CO3 + 2HCl → 2NaCl + H2O + CO2↑
- Calculation: 1 mole Na2CO3 ≡ 2 moles HCl.
Sulphuric Acid (H2SO4)
A secondary standard, similar to HCl. It is a strong diprotic acid. Concentrated H2SO4isusually 98% pure with a normality of 36 N or 18 M strength.
- Preparation (0.1 N):
- Strength = 98% and 36 N.
- Volume required = V1 = N2V2 / N1 = 0.1 × 1000 / 36 ≈ 2.8 mL
- For 0.1 N solution, slowly add approx. 2.8 mL of concentrated H2SO4 to a volumetric flask containing distilled water and make up the volume to 1000 mL. (Note: Always add acid to water, never water to acid., to prevent vigorous splashing due to the exothermic reaction.)
- Standardization:
- Primary Standard: Anhydrous Na2CO3 (dried at 270°C).
- Indicator: Methyl Orange (yellow to faint pink) or Methyl Red.
- Reaction: Na2CO3 + H2SO4 → Na2SO4 + H2O + CO2↑
- Calculation: 1 mole Na2CO3 ≡ 2 moles HCl.
Sodium Thiosulphate (Na2S2O3 ⋅ 5H2O)
Used primarily in iodometry. It is a secondary standard as it can be decomposed by bacteria, light, and atmospheric oxygen.
- Preparation (0.1 N):
- Molecular Weight = (2×23) + (2×32) + (3×16) + (5×18) = 46 + 64 + 48 + 90 = 248 g/mol
- Equivalent Weight = Molecular Weight/1 = 248.
- Dissolve approximately 25 g of Na2S2O3 ⋅ 5H2O and 0.2 g of Na2CO3 (preservative) in boiled, cooled distilled (CO₂-free) water and make up the volume to 1000 mL in a volumetric flask.
- Standardization (using Potassium Dichromate/Iodate):
- Principle: Iodometry. K2Cr2O7 reacts with KI to liberate I2, which is then titrated with thiosulphate.
- Indicator: Starch (added near the end point; color change from blue-black to colorless/light green).
- Reaction:
- Step 1 (Liberation): K2Cr2O7 + 14HCl + 6KI → 8KCl + 2CrCl3 + 3I2 + 7H2O
- Step 2 (Titration): I2 + 2Na2S2O3 → 2NaI + Na2S4O6 (Sodium Tetrathionate)
Potassium Permanganate (KMnO4)
- A powerful oxidizing agent, but not a primary standard due to the presence of MnO2 impurities. In acidic medium, Manganese is reduced from +7 to +2. For acidic medium H2SO4is used as it is stable in nature. KMnO4would oxidize Cl– to Cl2 gas, leading to an incorrect reading. HNO3 is itself an oxidizing agent and would interfere with the reaction. So HCl and HNO3 is not used.
- Preparation (0.1 N):
- Molecular Weight = 19 + 55 + (4 x 16) = 158.03 g/mol
- n-factor: 5 (change in oxidation state: 7 – 2 = 5)
- Equivalent Weight = Molecular Weight / 5= 31.6
- Dissolve 3.2 g in 1000 mL water. Boil for 15–30 mins, allow to stand for 24 hours, and filter through glass wool to remove MnO2. It is done to remove MnO2 which catalyzes further decomposition).
- Standardization (using Oxalic Acid):
- Principle: Redox titration in acidic medium (H2SO4).
- Indicator: Self-indicator (colorless to permanent pale pink).
- Condition: Heat the oxalic acid solution to 60–70°C before titration, to speed up the reaction.
- Reaction: 2KMnO4 + 5H2C2O4.5H2O + 3H2SO4 → K2SO4 + 2MnSO4 + 10CO2 + 18H2O.
- Ionic Equation: MnO4– + 8H+ + 5e– → Mn2+ + 4H2O (Reduction); C2O42- → 2CO2 + 2e– (Oxidation)
Ceric Ammonium Sulphate (Ce (NH4)4 (SO4)4 ⋅ 2H2O)
Used in ceriometry. It is very stable and a strong oxidizing agent. The Cerium ion in acidic media undergoes a one-electron change from Ce4+ (yellow) to Ce3+ (colorless). The titration is a Redox Titration using Ferroin as the indicator.
- Preparation (0.1 N):
- Molecular Weight = (Ce:140, S: 32, O:16, N: 14, H:1) = 632.55 g/mol
- n-factor: 1
- Equivalent Weight = Molecular Weight / 1= 632.55
- Dissolve 63.25 g of Ceric Ammonium Sulphate in a mixture of 30 mL H2SO4 and 500 mL water with gentle heat. Cool and dilute to 1000 mL with distilled water. Note: Acid is essential to prevent the hydrolysis of the Ceric ion into basic salts.
- Standardization (using Arsenic Trioxide):
- Primary Standard: As2O3. Its eq. wt. = 49.46. Weigh exactly 0.4946 g of As2O3. Dissolve it in 20 mL of 1 N NaOH by warming. Neutralize the excess base with dilute H2SO4 and make up to 100 mL.
- Indicator: Ferroin (Red to pale Blue).
- Catalyst: Osmic acid (to accelerate the reaction between Ce4+ and As3+).
- Reaction: 2Ce (SO4)2 + H3AsO3 + H2O → Ce2(SO4)3 + H3AsO4 + H2SO4 ; 2Ce4+ + As3+ → 2Ce3+ + As5+
Observation Table for titration
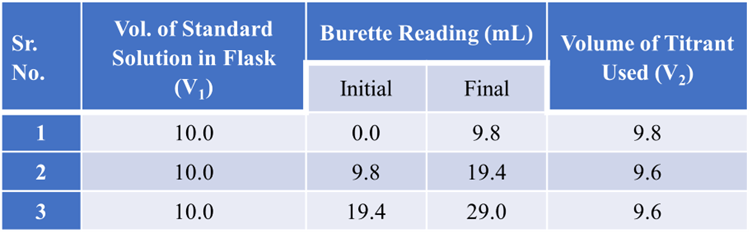
Summary Table for standardization of various solutions

End Point vs. Equivalence Point
What you see with your eyes (End Point) is slightly different from the perfect mathematical moment (Equivalence Point).

Tips for the Practical
- The Meniscus: For colorless solutions (NaOH, HCl), read the lower meniscus. For colored solutions (KMnO4), read the upper meniscus.
- Concordant Values: These are two or three burette readings that are exactly the same (e.g., 9.5, 9.5, 9.5). They ensure the precision and reliability of the experiment.
- Air Bubbles: Always check for air bubbles in the nozzle of the burette before starting, as they will lead to an incorrect volume reading.