In Pharmaceutical Chemistry, an impurity is any substance present in a medicinal agent other than the chemical entity itself. Even a tiny amount of impurity can make a drug toxic or reduce its shelf life. It is very essential to understand where these impurities come from for ensuring drug safety and quality.
Impurities can enter a drug at any stage—from the raw materials used to the way the final product is stored on a shelf. Here are the primary sources:
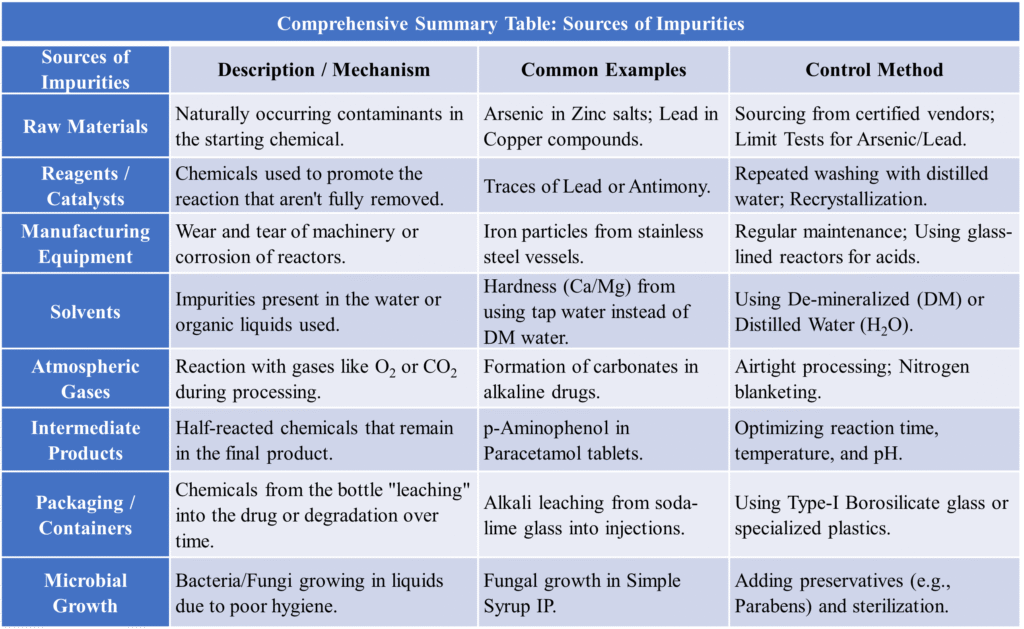
Raw Materials
Industries buy “Bulk Drugs” or “Active Pharmaceutical Ingredients” (APIs) and other substances as raw materials. If the supplier provides a lower grade of chemical, the impurity starts here. If these are not removed during the early stages, they carry over to the final product. Control: Performing “Identity and Purity” tests before the material enters the factory.
- Example: Rock salt used to make Sodium Chloride may contain traces of calcium and magnesium. When manufacturing Zinc Sulfate, the raw material Zinc metal often contains traces of Arsenic, Iron, and Lead, which can contaminate the final medicine.
Reagents and Catalysts
During chemical synthesis, various reagents or catalysts are added to speed up the reaction. Incomplete reactions or side reactions create unwanted by-products. If the final washing process is incomplete, these remain in the drug. Control: Maintaining precise temperature and pressure to ensure the reaction goes to completion.
- Example: Use of heavy metals like Mercury, Lead or Platinum as catalysts in industrial synthesis can leave toxic trace levels. In the preparation of Potassium Bromide, Barium is used to remove sulfate, which may leave Barium as a trace impurity.
Manufacturing Process
The drug (API) is mixed with “Excipients” (binders, colors, flavors) to make a tablet or syrup. The Formulation & Processing itself can introduce impurities through:
- Particulate Contamination: Dust, glass chips, or metal pieces from machines. Example: Iron particles can enter a product from the wear and tear of stainless-steel reactors. Control: Using high-grade Stainless Steel (316 grade) and regular equipment maintenance.
- Cross-contamination: When a previous batch of a different drug contaminates the current one. Example: Traces of Penicillin in a non-penicillin production line (highly dangerous due to allergies).
- Solvents: Water or organic solvents used for dissolving chemicals might contain dissolved gases or minerals. In the production of Alcohol, water used might introduce “Hardness” (calcium salts).
- Intermediate Products: Sometimes a reaction doesn’t go to 100% completion, leaving behind “intermediate” chemicals. Example: In the production of Paracetamol, a by-product called Diacetylated Paracetamol can form.
Chemical Incompatibility
If two substances react unexpectedly during the formulation, they create a new, unwanted impurity.
- Example: Certain active drugs react with “Excipients” (like lactose or starch) to form degradation products.
Purification (The Cleaning Stage)
This is where the industry removes the impurities. Techniques like Filtration, Distillation, and Crystallization are used.
- Example: Recrystallization is often used to remove traces of reagents from the final drug crystals.
Atmospheric Contamination
During the manufacturing process, the drug is exposed to the air. It can pick up dust, smoke, or gases like Carbon Dioxide and Sulfur Dioxide.
- Example: Sodium Hydroxide absorbs CO2 from the atmosphere to form Sodium Carbonate.
Packaging & Quality Control (Leaching from Containers)
The drug is placed in its final container (blister pack, glass vial). If a drug is stored in poor-quality glass or plastic, the chemicals from the container can “leach” into the medicine. Impurity Point: Environmental dust or microbial growth during filling. It is controlled by using HEPA filters in the air and conducting the final Limit Tests according to the Pharmacopoeia.
- Example: Alkali can leach from cheap soda-glass bottles into liquid injections, changing the pH.
Decomposition on Storage
Some drugs are unstable and break down over time due to light, heat, or moisture.
- Example: Aspirin (Acetylsalicylic acid) breaks down into Salicylic acid and acetic acid when exposed to moisture.
To ensure that medicinal agents are free from the sources of impurities discussed above, the pharmaceutical industries strictly follow Current Good Manufacturing Practices (cGMP) and perform Limit Tests as prescribed by the Indian Pharmacopoeia (IP).
Comprehensive Summary Table: Sources of Impurities
Chemical Degradation Pathways
Impurities are often formed when a drug or its starting material undergoes a chemical change. Here are the most common chemical-based reactions responsible for impurities, explained with simple equations.
Hydrolytic Degradation / Hydrolysis (Reaction with water)
This occurs when a drug reacts with water molecules, often breaking a chemical bond. This is the most common cause of drug degradation. Many drugs contain “Ester” or “Amide” groups that break down when exposed to moisture in the air.
Example: Aspirin. When Aspirin (Acetylsalicylic acid) is stored in a humid environment, it reacts with water to form toxic by-products like Salicylic Acid and Acetic Acid. This is why old aspirin bottles smell like vinegar. It reduces the potency of the drug.
C9H8O4 (Aspirin) + H2O → C7H6O3 (Salicylic Acid) + CH3COOH (Acetic Acid)
Oxidation (Reaction with Oxygen)
Oxidation involves the loss of electrons or the addition of oxygen. It is often triggered by light (photolysis), heat, or trace metal ions (like Iron or Copper). Some drugs are sensitive to atmospheric oxygen. This is why “Antioxidants” are added to many medicinal agents to prevent color change and loss of activity.
Example: Ferrous Sulfate. When green Ferrous Sulfate (Fe2+) is exposed to moist air, it oxidizes to brown Ferric Sulfate (Fe3+). This makes the drug ineffective.
4FeSO4 (Ferrous Sulfate) + 2H2O + O2 → 4Fe(OH)SO4 (Basic Ferric Sulfate)
Atmospheric Carbonation (Reaction with CO2)
Highly alkaline drugs can absorb Carbon Dioxide from the atmosphere to form carbonate impurities. Absorption of CO2 converts hydroxides into carbonates.
Example: Sodium Hydroxide. When NaOH is left exposed, it reacts with CO2 to form Sodium Carbonate.
2NaOH (Sodium Hydroxide) + CO2 → Na2CO3 (Sodium Carbonate) + H2O
Synthesis-Related Side Reactions
Sometimes during the manufacturing of a drug, the chemical reaction goes “too far,” or reacts at the wrong site, creating a by-product impurity. Incomplete reduction or over-reaction during the bulk drug synthesis cause impurity.
Example: Paracetamol Synthesis. Paracetamol is made by reacting p-aminophenol with acetic anhydride. If an over-reaction occurs, an impurity called Diacetylated Paracetamol (4-acetamidophenyl acetate) is formed.
Another Example: Potassium Iodide (KI) In the production of KI, an intermediate called Potassium Iodate (KIO3) is formed. If it is not fully reduced by charcoal, it remains as a toxic impurity.
KIO3 (Potassium Iodate) + 3C (charcoal) → KI (Potassium Iodide) + 3CO ↑
Summary Table for Reactions
