Stereochemistry is the ‘chemistry of space’. It deals with the spatial arrangements of atoms and groups in a molecule. Stereoisomerism is crucial to understand because the spatial arrangement of atoms can completely change a drug’s effect: one isomer might cure a disease, while its “mirror image” could be toxic. It is essential to know about stereoisomerism because the three-dimensional “shape” of a drug molecule often determines its biological activity, safety, and metabolism.
Definition
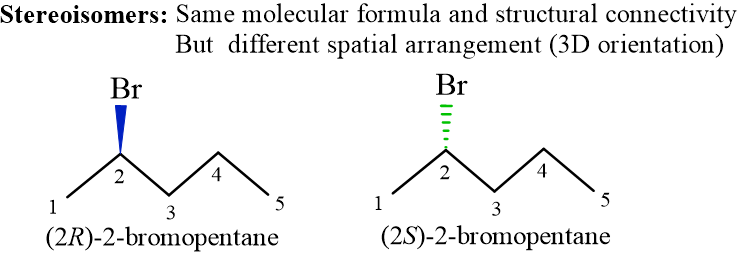
Stereoisomers are compounds that have the same molecular formula and structural connectivity (atoms are bonded in the same order), but they differ in the spatial arrangement (3D orientation) of their atoms in space. Key Difference: Unlike structural isomers (like butane vs. isobutane), stereoisomers have the same “skeleton” but different “3D shapes.”
Classification
Stereoisomerism is broadly divided into two main categories:
Conformational Isomerism
These isomers can be interconverted by rotation around a single bond (C-C) without breaking any bonds. They are usually interconvertible at room temperature and cannot be easily separated. Example: Conformations of Ethane (Staggered and Eclipsed).
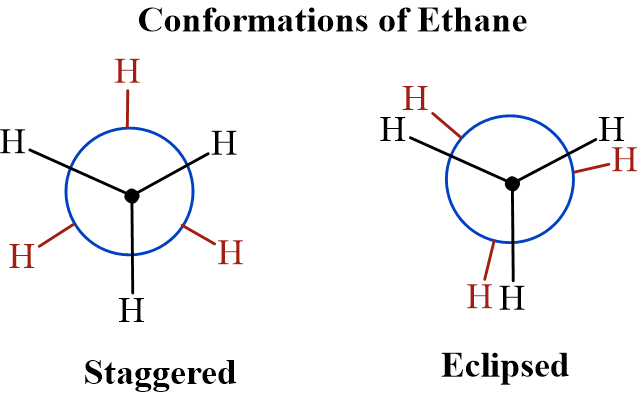
Significance: Many drugs change their “conformation” to fit into a receptor, known as the “active conformation.”
Configurational Isomerism
These isomers can only be interconverted by breaking and reforming chemical bonds. They are further subdivided into:
Geometrical Isomerism (Cis-Trans Isomerism)
This occurs due to restricted rotation around a double bond (C=C) or in cyclic structures.
Cis-Isomer: Similar groups are on the same side. Example: Maleic Acid.
Trans-Isomer: Similar groups are on the opposite side. Example: Fumaric Acid.
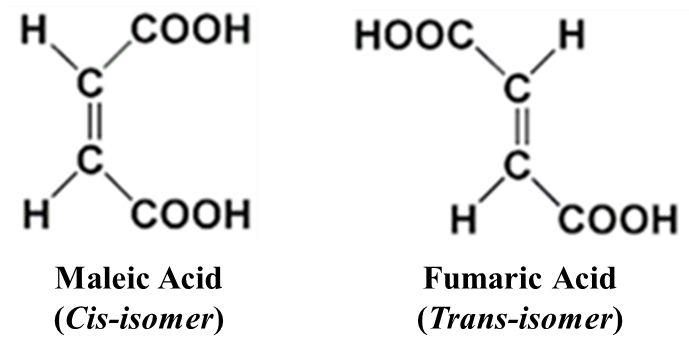
Maleic Acid (Cis): Both -COOH groups are on the same side. It is toxic and less stable due to steric hindrance. It has a lower melting point.
Fumaric Acid (Trans): The -COOH groups are on opposite sides. It is more stable, occurs naturally in the skin during sunlight exposure and has a higher melting point.
Optical Isomerism
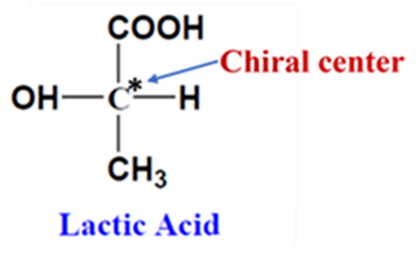
This is the most “pharmaceutically active” form of isomerism. This occurs in molecules that contain a chiral center (a carbon atom bonded to four different groups). These molecules rotate the Plane Polarized Light (PPL).
Enantiomers (Mirror Images)
Non-superimposable mirror images that cannot be covered perfectly on top of each other (like your left and right hands). They have identical physical properties (BP, MP) but different biological effects.
Example: Lactic Acid, it has one chiral center. It exists as:
(+)-Lactic acid: Rotates light to the right (found in muscle tissue).
(-)-Lactic acid: Rotates light to the left (found in sour milk).
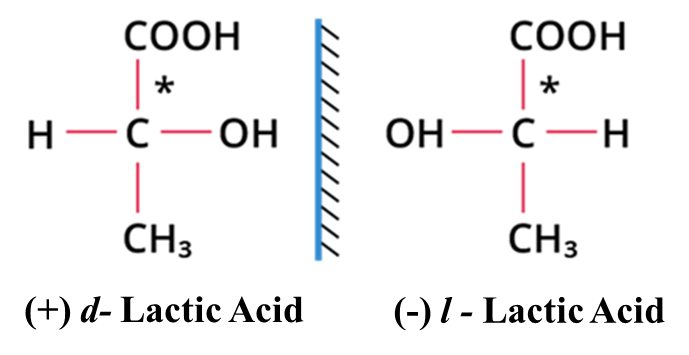
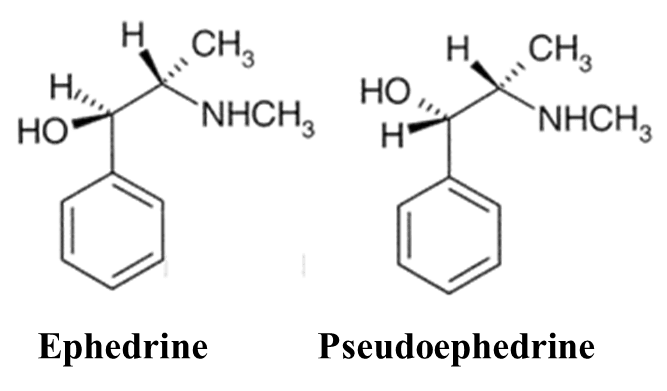
Diastereomers (Non-Mirror Images)
Stereoisomers that are not mirror images of each other. They occur when a molecule has two or more chiral centers. Example: Ephedrine and Pseudoephedrine
Ephedrine: Used to treat low blood pressure.
Pseudoephedrine: Used as a nasal decongestant.
They have the same formula but different 3D shapes that are not mirror images, leading to different medicinal uses.
Pharmaceutical Significance (The “Clinical” Angle)
Receptors in the body are chiral (like a “lock“), and only the correct “key” (isomer) fits. These isomers have the significance biological impact.
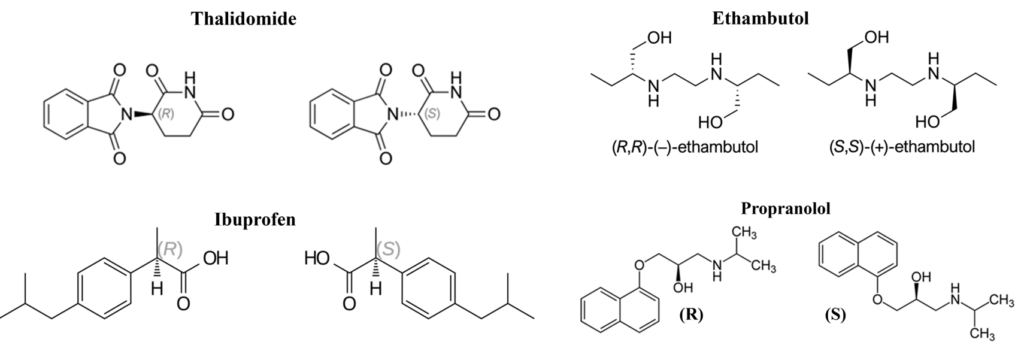
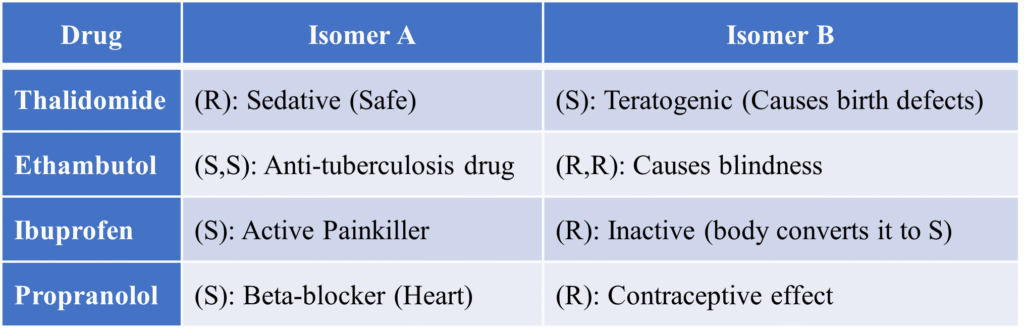
The thalidomide disaster
In the 1950s and 60s it was a haunting example of how a lack of understanding in molecular chirality can lead to global catastrophe. It struck Germany in the year 1957. Originally sold as an over-the-counter sedative and treatment for morning sickness, Thalidomide resulted in over 5,000 babies being born with severe limb deformities (phocomelia).
Ethambutol (The Vision Danger)
Ethambutol is a primary medication used to treat Tuberculosis (TB). However, its stereochemistry is a matter of “life and sight.” The (S,S)-Enantiomer (Eutomer) is the therapeutically active form. It effectively inhibits the growth of the TB bacteria (Mycobacterium tuberculosis). The (R,R)-Enantiomer (Distomer) is highly toxic. It causes optic neuritis, which leads to permanent blindness (loss of vision and color discrimination).
Ibuprofen (The Metabolic Switch)
Ibuprofen is a Non-Steroidal Anti-Inflammatory Drug (NSAID) used for pain and fever. It presents a fascinating case of in vivo chiral inversion. The (S)-Enantiomer is the pharmacologically active form that inhibits the COX enzyme to reduce pain and inflammation. The (R)-Enantiomer form is considered inactive in the test tube (in vitro). Significance: Interestingly, the human body contains an enzyme (isomerase) that converts the “inactive” (R)-form into the “active” (S)-form.
Propranolol (The Selective Blocker)
Propranolol is a “Beta-blocker” used to treat high blood pressure, chest pain (angina), and heart rhythm disorders. The (S)-Enantiomer is the active heart medication. It is 100 times more potent than the (R)-isomer at blocking beta-receptors in the heart. The (R)-Enantiomer doesn’t affect the heart much. It inhibits the conversion of thyroid hormones. In some historical contexts, it was even studied for its contraceptive properties. Significance: Propranolol is usually administered as a racemic mixture, even though the (S)-isomer does all the heavy lifting for heart health.