Optical activity is the ability of a substance to rotate the plane of Plane Polarized Light (PPL). This occurs in molecules that contain a chiral center (a carbon atom bonded to four different groups). This phenomenon is a cornerstone of stereochemistry and is vital in pharmaceutical analysis for identifying drugs and checking their purity.
The Core Mechanism: How it Works
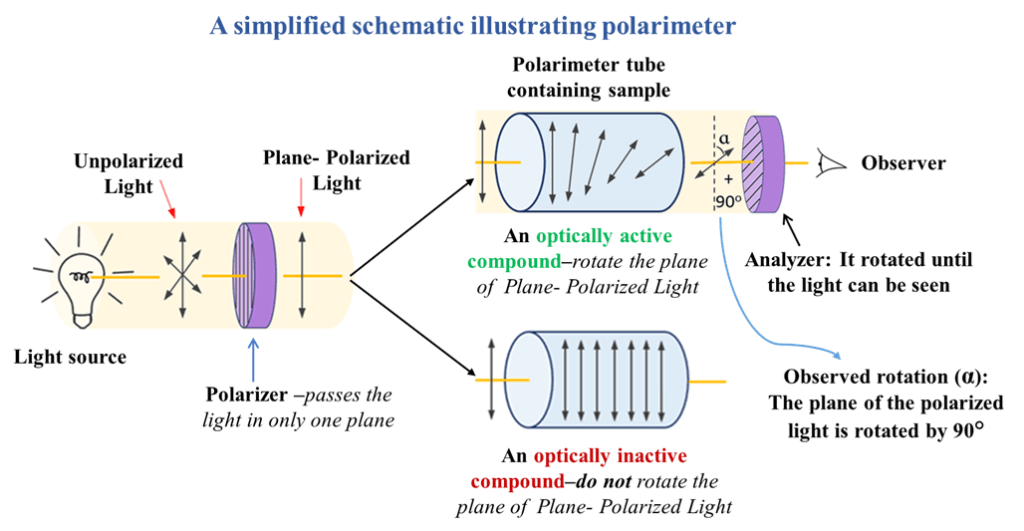
To understand optical activity, we must follow the journey of light through a Polarimeter (the instrument used to measure it).
- Step 1: Ordinary Light: Normal light vibrates in all possible planes perpendicular to its direction of travel.
- Step 2: Polarization: This light is passed through a Nicol Prism (made of calcite) or a polarizing filter. This filter blocks all vibrations except those in one single plane. This is now Plane Polarized Light (PPL).
- Step 3: Interaction with Sample: When PPL passes through a solution of an optically active substance (like glucose or a chiral drug), the “electric fields” of the chiral molecules interact with the light, causing the plane of vibration to “twist” or rotate.
- Step 4: Detection: An observer uses a second prism (the Analyzer) to measure the degree of this rotation.
Direction of Rotation
Optical activity is classified based on which way the light is turned:
- Dextrorotatory (+ or d):
- The substance rotates the plane of PPL to the right (clockwise).
- Example: (+)-Glucose is often called Dextrose.
- Levorotatory (- or l):
- The substance rotates the plane of PPL to the left (counter-clockwise).
- Example: (-)-Fructose is often called Levulose.
Important Note: The direction of rotation (+ or -) has no relationship to the R/S configuration. (+/-) is determined only by experiment while R/S is determined by structure (rules).
Specific Rotation [α]
Since the amount of rotation depends on how much sample the light hits, we use a standardized value called Specific Rotation to compare different substances. The specific rotation of a molecule is the rotation in degrees observed upon passing polarized light through a path length of 1 decimetre (dm) at a concentration of 1 g/mL.
The formula for specific rotation is as below:
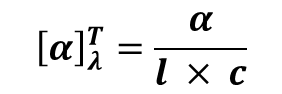

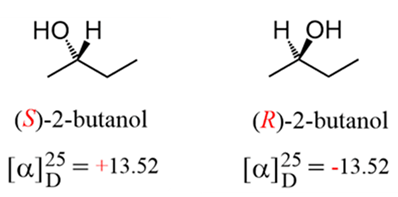
Example: Suppose the experiment was run at the temperature of 25 °C using D line of a sodium lamp (λ = 589.6 nm). An optically active sample (2-butanol) with a concentration of 1.00 g/mL was placed in a 1 dm polarimeter tube. It resulted in a rotation of 13.52° in a clockwise direction and it was called as the specific rotation of 2-butanol.
Examples of Specific Rotation in Drugs
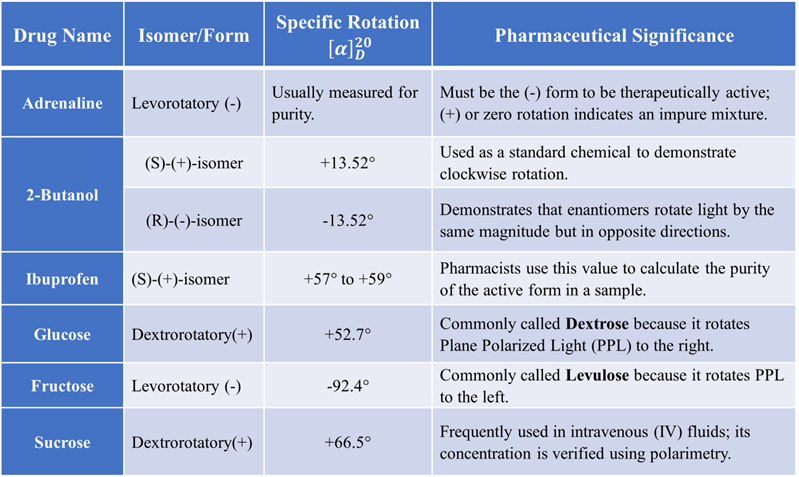
Be sure to remember that there is NO relationship between the R and S configuration and the d (+) and l (–) notations. The R and S notation is used for labeling the absolute configuration of chirality centers while the d (+) and l (–) notations indicate the direction to which the sample rotate the plane of a polarize light.
Significance in Pharmacy
In pharmaceutical sciences, optical activity is more than just a physical property. It is a critical quality control parameter and a legal requirement for drug standards. If a drug is optically active, its specific rotation must be documented in official pharmacopoeias (like IP, BP, or USP).
Identification and Fingerprinting
Every optically active drug has a unique Specific Rotation (α) value. In a quality control (QC) lab, pharmacists use a polarimeter to verify the identity of a raw material.
Example: If you receive a shipment of Adrenaline, it must be the levorotatory (-) form. If the polarimeter shows a (+) rotation or zero rotation, the sample is either the wrong isomer or an impure mixture.
Determination of Optical Purity (Enantiomeric Excess)
Many drugs are manufactured as single enantiomers because the “other half” might be toxic or inactive. Optical activity helps determine how “pure” the chiral drug is. A Racemic Mixture (50:50 of + and -) has zero optical activity (due to external compensation).
If a sample of (S)-Ibuprofen shows less rotation than the standard pure (S)-Ibuprofen, the pharmacist can calculate exactly how much of the “inactive” (R)-isomer is contaminating the batch.
Monitoring Drug Stability and Shelf Life
Some drugs undergo Racemization over time. This is a process where a pure, active enantiomer slowly converts into an inactive mixture due to heat, light, or pH changes.
Example: Pilocarpine (used for glaucoma) can lose its activity if it racemizes. By measuring optical activity during stability testing, pharmacists determine the expiration date of a medicine.
Quantitative Analysis (Assay)
For certain clear solutions, the degree of rotation is directly proportional to the concentration of the drug.
Example: In the sugar industry or pharmaceutical manufacturing, the concentration of sucrose or dextrose in intravenous (IV) fluids is often checked using polarimetry. The degree of rotation is used to calculate the exact concentration of a syrup or solution.
Summary of Optical Activity in Pharmaceutical Sciences