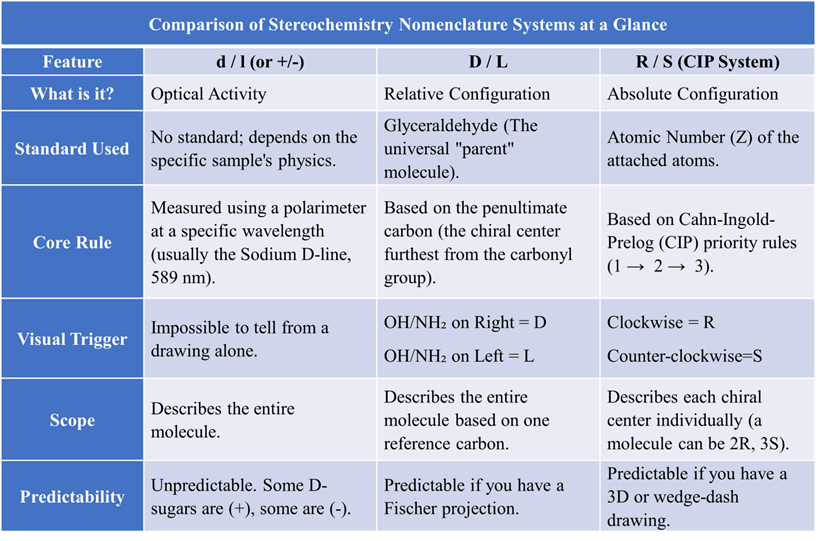
Stereochemistry can feel like learning three different “languages” to describe the same object. There are different stereochemistry nomenclature system for organic compounds. One language describes how it behaves in light, one describes its family history, and the last describes its exact physical shape.
Here is a simplified, comprehensive breakdown of these systems.
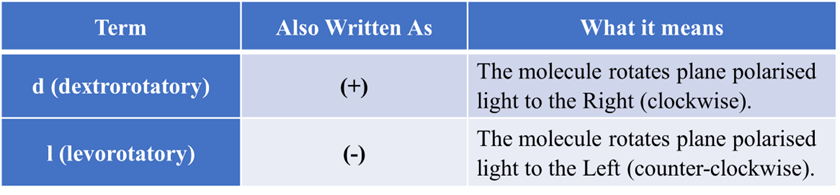
The “Light” System: d(+) and l(-)
This system is experimental. You cannot tell if a molecule is (+) or (-) just by looking at a drawing; you have to put it in a machine called a polarimeter and see what happens.
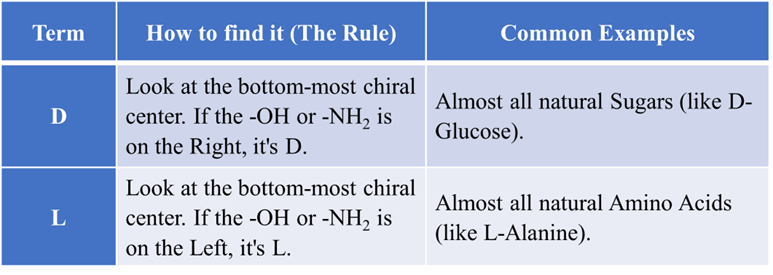
The “Family” System: D and L
This is an old system used mostly for sugars and amino acids. It compares the molecule to a “standard” (Glyceraldehyde). It is based on what the molecule looks like in a flat drawing called a Fischer Projection.
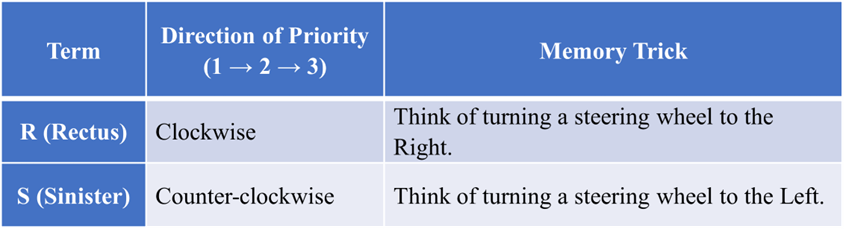
The “GPS” System: R and S
This is the modern, absolute way to describe a molecule’s shape. It uses the “CIP Rules” (ranking atoms by their atomic numbers). It is like giving a molecule a precise GPS coordinate.
The Golden Rule: These systems are independent! A molecule can be D (family), (+) (light), and (S) (geometry) all at the same time. One does not automatically tell you the other.
Comparison of Stereochemistry Nomenclature Systems at a Glance