Introduction
The Acid Value (AV) is one of the most important tests in food science and industrial chemistry. It essentially measures how “spoiled” or “aged” an edible fat and oil is. It tells us if an oil is fresh, if it is suitable for a specific purpose like cooking or soap-making or if it has been mixed with cheaper oils (adulteration).
Theory
Fats and oils are triglycerides (three fatty acids attached to a glycerol backbone). When oil is fresh, these fatty acids are “locked” inside the molecule.
However, as oil stored for a long time or is exposed to heat and moisture, the bonds break due to oxidation. This process is called hydrolysis. It releases “Free Fatty Acids” (FFAs). The more refined or processed the oil is, the higher the free fatty acid content. In general, the acid value increases with the age of an oil as triglycerides decompose into smaller fatty acids and glycerol as an effect of time.
Principle
The test is based on a simple neutralization reaction (Acid + Base → Salt + Water). We take the acidic oil and add a base (Potassium Hydroxide, KOH) until the acid is neutralized. For solvent choice, we use a mixture of Ethanol/Diethyl Ether to ensure the fat dissolves completely so the base (KOH) can react with the acids.
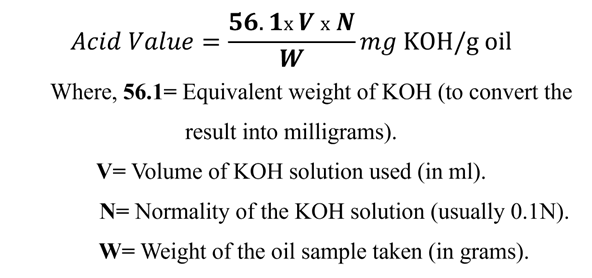
Definition: The Acid Value is the number of milligrams (mg) of KOH required to neutralize the free fatty acids present in 1 gram of the fat or oil.
Chemical Reaction
The free fatty acid (represented as R-COOH) reacts with the KOH as follows:
R-COOH + KOH → R-COOK + H2O
(Free Fatty Acid + Potassium Hydroxide → Potassium Salt of Fatty Acid + Water)
Formula
To calculate the Acid Value, we use the results from the titration and apply the following formula:
Procedure
- Preparation: Weigh a specific amount of oil (e.g., 5g to 10g) into a conical flask.
- Solvent: Add about 50 ml of a neutral solvent (usually a mixture of Ethanol and Diethyl Ether) into a conical flask. The solvent must be “neutral” so its own acidity doesn’t ruin the test.
- Indicator: Add 2–3 drops of Phenolphthalein. The solution will remain colorless because the oil is acidic.
- Titration: Slowly add standard 0.1N KOH from a burette while shaking the conical flask constantly.
- End Point: Stop when a faint pink color appears and persists for at least 15 seconds. Note the volume (V) of KOH used.
- Calculate the Acid Value as per the given formula.
Significance
- Freshness: A low acid value means the oil is fresh and has been processed well.
- Rancidity: A high acid value indicates the oil is old or has been stored under poor conditions (moisture/heat). The oil becomes “rancid,” and might taste or smell bad.
- Industrial Use: In soap making, knowing the acid value helps determine how much alkali is needed.
- Edibility: If the acid value is too high, the oil is considered unfit for human consumption.
Real-World Examples
| Oil Type | Typical Acid Value (mg KOH/g oil) | Status |
| Refined Sunflower Oil | 0.1 to 0.5 | Very Fresh / High Quality |
| Cold-Pressed Olive Oil | 0.5 to 1.5 | Normal for unrefined oil |
| Used Frying Oil | 5.0 to 10.0 | Spoiled / Degraded (Throw away) |
| Crude Palm Oil | >10.0 | Needs heavy refining before eating |
Practice Problem
A quality control chemist weighs 10.0 g of an old mustard oil sample. During titration, it requires 2.4 ml of 0.1 N KOH to reach the phenolphthalein end-point. Calculate the Acid Value.
Solution
Formula:
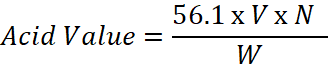
Calculation:
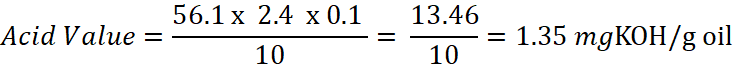
Interpretation: This is a relatively low acid value, suggesting the oil is still quite fresh.
Master Revision Table: Analytical Constants
| Parameter | What it Measures | Principal Reagent | Key Sample/Limit (IP) |
| Acid Value | Rancidity (Free Fatty Acids) | 0.1M KOH | Generally, < 2.0 for purity. |
| Saponification Value | Chain length / Molecular Weight | Alcoholic KOH | Coconut Oil: 250–264 (Highest) |
| Ester Value | Bound Fatty Acids | (Derived) | Beeswax: 70–80 |
| Acetyl Value | Free Hydroxyl (—OH) Groups | Acetic Anhydride | Castor Oil: more than 143 |
| Iodine Value | Double Bonds (-C=C-), Degree of Unsaturation | Wijs Reagent (ICl) | Unsaturated oils, Castor Oil: 82–90 Linseed Oil: >170 |