Baeyer Strain Theory explains why some rings of carbon atoms (cycloalkanes) are very stable while others are “unhappy” and want to break open. The stability of cycloalkanes is explained by the concept of ring strain. It is the total strain energy stored in a cyclic molecule due to its geometry. The main contributions to ring strain are angle strain, torsional strain, and steric strain.
Imagine you have a set of flexible sticks. Some shapes you build with them feel natural and strong, while others feel like they are about to be broken suddenly. Adolf von Baeyer in 1885 proposed the first theory for that “tension” to explain the stability of small cycloalkanes.
- The “Perfect” Angle: To understand the theory, you first need to know what carbon likes. In a normal chain (like propane), a carbon atom wants its bonds to be at an angle of 109.5°. It is the “comfort zone.” If you force the bonds to be closer or further apart, the molecule feels Angle Strain.
- The Core Idea: Baeyer proposed that when carbon atoms form a ring, they are forced out of their 109.5° comfort zone to fit the shape of a polygon (triangle, square, etc.). The further away the angle is from 109.5°, the more “strained” and unstable the ring becomes.
Baeyer’s Postulate
- Planar Rings: Baeyer assumed that all cycloalkane rings are planar (flat).
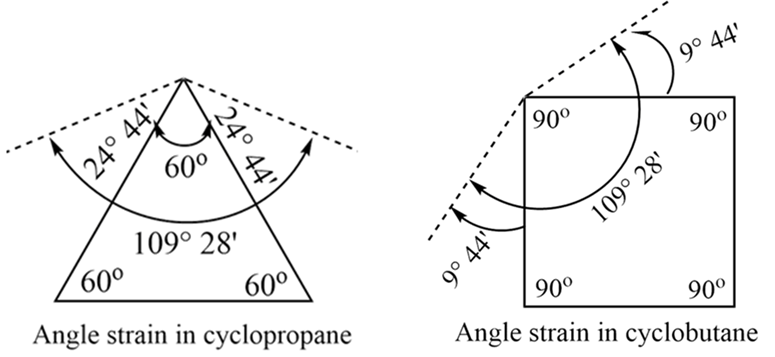
- Angle Strain: Cycloalkanes are strained because their C-C-C bond angles (α) are forced to deviate from the ideal tetrahedral angle of 1090 28′ (109.50).
- Stability: The greater the deviation from the ideal angle, the greater the angle strain, and the less stable the cycloalkane. This is why cyclopropane is highly reactive—it wants to break the ring to “relax.”
The degree of deviation (d) is calculated as:

The internal angle (α) of a regular polygon is calculated as :
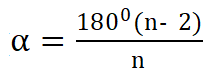
where n is the number of sides/carbons
The angle strain in cyclopropane and cyclobutane is shown as below:
The Stability Rankings (According to Baeyer) is given in following table:
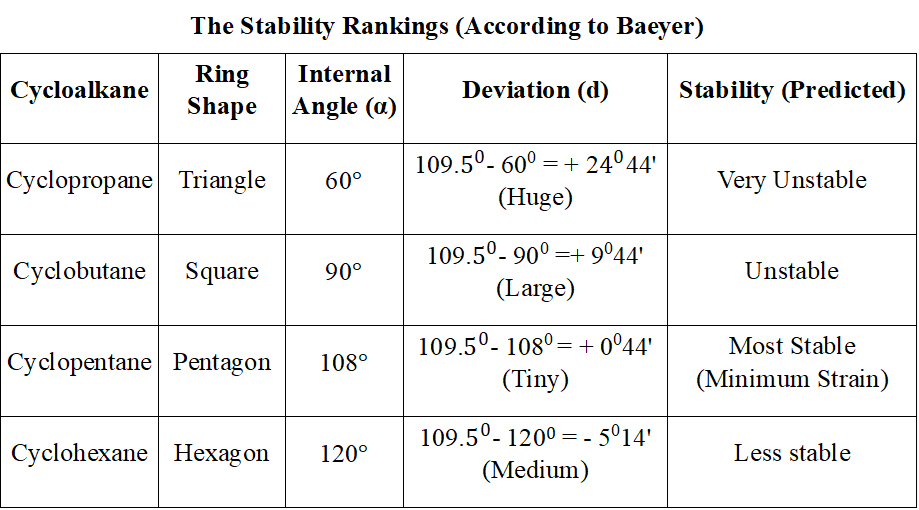
- Based on above calculation, Baeyer predicted the stability order as below:
- Cyclopentane > Cyclobutane > Cyclopropane > Cyclohexane (and higher rings)
Limitations of Baeyer’s Strain Theory:
Baeyer made one big mistake. He assumed all rings are flat (planar). Because he thought they were flat, he predicted that as rings got bigger (like Cyclohexane or Cyclooctane), they would get more unstable because the angles would keep getting wider. Experimental data (specifically, Heat of Combustion values per CH2 group, which is a measure of strain) contradicted Baeyer’s predictions for larger rings.
- Heat of Combustion values per CH2 group: It is a measure of strain contradicted Baeyer’s predictions for larger rings. It is the amount of heat evolved, when one mole of that compound is completely burnt to CO2 and H2O per CH2. Heat of combustion per CH2 group in:
- Cyclopropane = 697 kJ/mol
- Cyclobutane = 686 kJ/mol
- Cyclopentane = 664 kJ/mol
- Cyclohexane = 658·5 kJ/mol
- Open-chain alkanes =658·5 kJ/mol
Cyclopropane and Cyclobutane contain more energy per CH2 group than cyclopentane or cyclohexane or open-chain alkanes. Consequently, cyclopropane and cyclobutane should be less stable than cyclopentane or cyclohexane. Such data on the heat of combustion of cycloalkanes would suggest that all cycloalkanes containing more than Five carbon atoms have stability similar to that of open-chain alkanes. If Baeyer ‘s theory is correct, heat of combustion should increase steadily with the ring size.
- Incorrect Stability Order: Experimentally, Cyclohexane is the most stable of the common cycloalkanes, and the stability order is: Cyclohexane > Cyclopentane > Cyclobutane > Cyclopropane.
- Larger Rings are Stable: Baeyer predicted that rings larger than cyclopentane (e.g., cyclohexane, cycloheptane, cyclooctane) would have an increasing negative angle strain (bond angles > 109.50) and thus be increasingly unstable. In reality, large rings aren’t flat! They “pucker” or fold (like a lawn chair) to keep their angles at exactly 109.5°. Cyclohexane is actually more stable than Cyclopentane because it folds into a “chair” shape to eliminate all strain.
- Incorrect Planarity Assumption: The core limitation is the assumption that all rings are planar. As discovered later, medium and large rings are non-planar (puckered) to relieve angle strain.
Summary:
- Carbon loves 109.5°.
- Small rings (3 or 4 carbons) are unstable because they are “squeezed.”
- Cyclopentane was Baeyer’s “gold standard” for stability.
- Big rings cheat the system by folding themselves into 3D shapes to stay stable.