Introduction
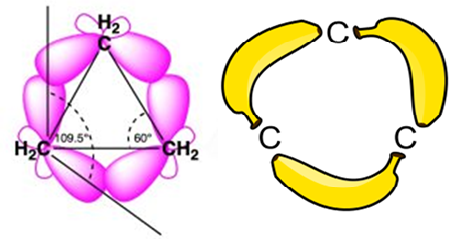
Baeyer’s Strain Theory assumed that bonds are straight lines between nuclei. Coulson and Moffitt (1947) corrected this by applying quantum mechanics, proving that in small rings like cyclopropane, bonds are actually curved.
The Concept of Bent (Banana) Bonds
In a normal alkane, carbon uses sp3 hybrid orbitals to form bonds. For maximum strength, these orbitals must point directly at each other (head-on overlap).
- The Conflict: In cyclopropane, the carbons form an equilateral triangle with 600 angles. However, sp3 orbitals naturally want to be at 109.50.
- The Solution: The orbitals cannot point directly at each other. Instead, they point slightly outside the triangle. When they overlap, they form a “curved” path of electron density and form poor orbital alignment.
Because this curved path resembles the shape of a banana, these are famously called Banana Bonds.
Detailed Features of the Modification
1. Re-hybridization (The sp5 Model)
Hybridization itself is a theoretical concept used to explain observed molecular shapes and properties. It is a mathematical model rather than a “real” physical phenomenon that can be directly observed spectroscopically. The sp5 model is an application of advanced bonding theory (like Coulson’s Theorem or Bent’s Rule) to describe a complex, strained system where the simple sp, sp², sp³ model is insufficient.
Coulson and Moffitt showed that the carbon atoms in cyclopropane don’t use “pure” sp3 orbitals. To reduce strain, the carbon redistributes its “s” and “p” character:
- C-C Bonds: Use more “p-character” (roughly sp5). Higher p-character allows the bond to be more flexible and “bendable.”
- C-H Bonds: Use more “s-character” (roughly sp2). This makes the C-H bonds shorter and stronger.
| Summary Table: sp3 vs. sp5 | ||
| Feature | Normal Alkane (sp3) | Cyclopropane (sp5 model) |
| S-Character in C-C | 25% | ~17% |
| P-Character in C-C | 75% | ~83% (Higher flexibility) |
| Bond Shape | Straight | Bent (Banana) |
| C-H Bond Character | sp3 | sp2 (More acidic/stronger) |
| Inter-orbital Angle | 109.50 | 1040 |
2. The Inter-orbital Angle
While the geometric angle (the corners of the triangle) is 600, the angle between the hybrid orbitals (the “starting” angle of the bonds) is actually about 1040. This is much closer to the ideal 109.50, which helps explain why cyclopropane can exist at all despite the massive strain predicted by Baeyer.
Real Examples and Evidence
A. Chemical Reactivity (The “Alkene” Behavior)
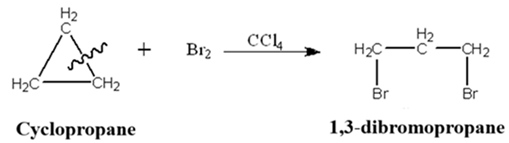
Because banana bonds have poor overlap and high electron density on the outside of the ring, they behave more like double bonds (π bonds) than single bonds (σ bonds).
Due to this enhanced p-character and significant ring strain, cyclopropanes can undergo reactions similar to alkenes, such as ring-opening and electrophilic addition reactions.
Example: Normal alkanes (like propane) don’t react with Br2 in the dark. However, cyclopropane undergoes a “ring-opening” reaction with Br2 in dark and form addition product 1,3-dibromopropane.
B. Physical Evidence (X-Ray Diffraction)
Advanced X-ray studies have mapped the actual electron density in cyclopropane. These maps show that the highest concentration of electrons is not on the straight line between the carbon nuclei but is concentrated outside the direct C-C internuclear axis. Thus, it provides experimental evidence for the long-theorized “bent bond” or “banana bond” model.
C. Bond Lengths
In a typical alkane, the C-C bond length is about 1.52 Å (152 pm). In cyclopropane, the “straight-line” distance between carbons is shorter 1.51 Å (151 pm). This is because the bent nature of the bonds pulls the nuclei closer together despite the repulsive strain.
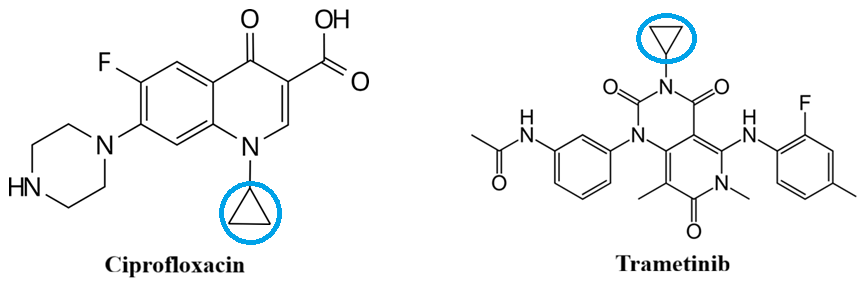
D. Drug Design (Medicinal Chemistry)
Cyclopropane rings are often used in drugs to “mimic” the electronics of a double bond while keeping the molecule more rigid. This makes the cyclopropyl group a useful bioisostere in medicinal chemistry. The C–H bonds in cyclopropanes are shorter and stronger than typical C–H bonds, making them less susceptible to oxidative metabolism by liver enzymes.
Example: In many enzyme inhibitors, a cyclopropane ring is swapped in place of a C=C double bond. The “banana bonds” provide a similar electronic environment for the enzyme to recognize, but the ring prevents the molecule from being metabolized as easily as a double bond would be.
Examples of drugs that utilize the cyclopropane moiety include the antibiotic Ciprofloxacin and the anticancer agent Trametinib, where the ring contributes to high potency and improved properties.