Introduction
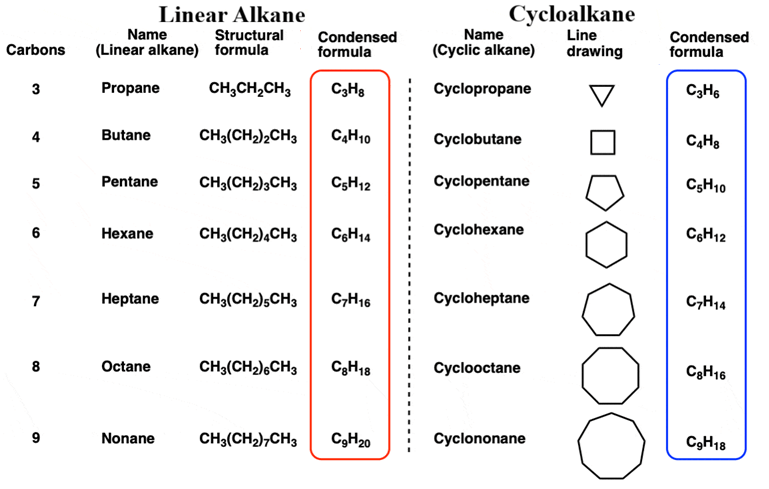
Cycloalkanes, also known as cycloparaffins, are saturated hydrocarbons in which the carbon atoms are joined together in a ring or cyclic structure. Since they are “saturated,” every carbon atom is connected by single bonds (sigma bonds) and carries the maximum possible number of hydrogen atoms.
The general formula for a cycloalkane is CnH2n (where “n” is the number of carbons). This is different from straight-chain alkanes (CnH2n+2) because the two ends of the carbon chain must “lose” two hydrogen atoms to join and form the ring.
Cycloalkanes contain a number of methylene groups(-CH2-) linked together to form a ring. Therefore, also named as polymethylenes.
Cyclic compounds are widely distributed in nature. Steroids, terpenoids, carbohydrates, antibiotics and nucleotides (DNA and RNA) are all cyclic natural products.
Cyclopropane (C3H6) is the smallest member. All carbon atoms are sp3 hybridized. In a perfect tetrahedral world, they would have bond angles of 109.50, but the ring shape often forces them into different angles, causing “ring strain.” The first few members (cyclopropane, cyclobutane) are gases at room temperature, while higher members are liquids or solids.
Classification
Cycloalkanes are typically classified based on the number of carbon atoms in the ring, which significantly influences their stability and reactivity.
| Class | Ring Size (n) | Examples | Stability Note |
| Small Rings | 3 to 4 | Cyclopropane, Cyclobutane | Highly strained and reactive. |
| Common Rings | 5 to 7 | Cyclopentane, Cyclohexane, Cycloheptane | Very stable; cyclohexane is the most stable. |
| Medium Rings | 8 to 11 | Cyclooctane, Cyclononane | Moderate stability; some “puckering” occurs. |
| Large Rings | 12+ | Cyclododecane | Very flexible and nearly strain-free. |
Preparation of cycloalkane
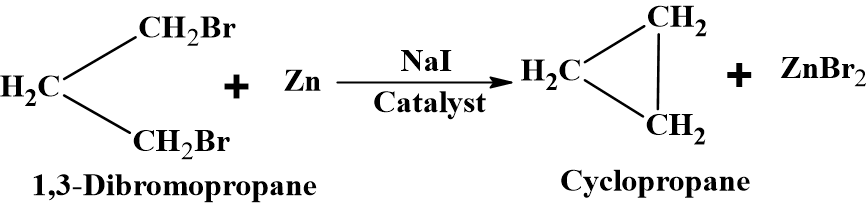
From dihalogen derivative of alkane
Freund’s Reaction: (Intramolecular Wurtz reaction)
A dihalogen compound containing halogens on terminal positions, on treatment with Zn, in the presence of NaI catalysts gets dehalogenated. The terminal halogen atoms are removed thereby forming the ring. This method is an extension of Wurtz reaction. This reaction is useful for the synthesis of 3 to 6 membered rings.

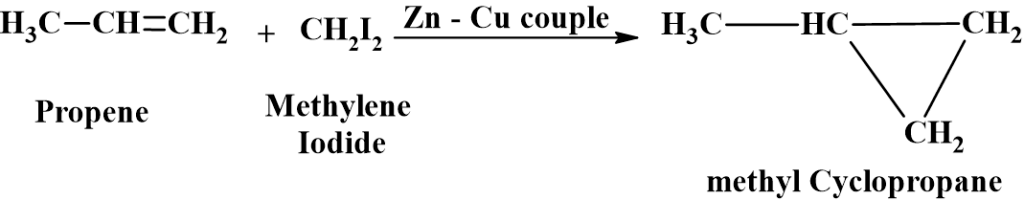
From Alkenes (Simmons-Smith Reaction)
Alkenes react with methylene iodide (CH2I2) in presence of Zn-Cu couple to form cyclopropane derivatives.
Reduction of aromatic compounds
Benzene and its derivatives can be catalytically hydrogenated by passing hydrogen gas and using Ni catalyst at high temperature (150-200oC) to produce cyclohexane and substituted cyclohexanes.
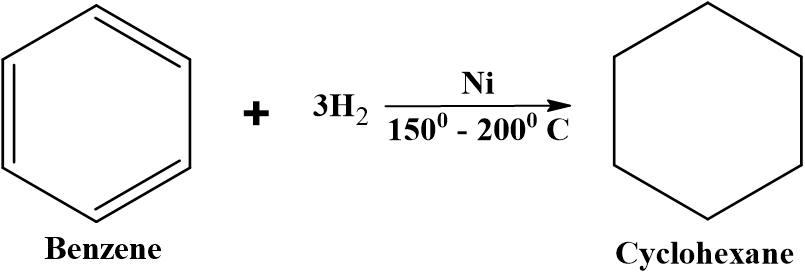
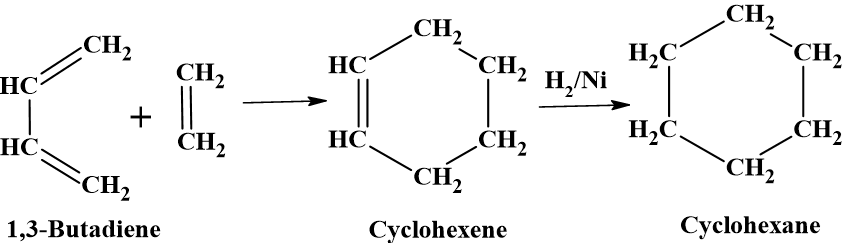
Dields-Alder reaction
The (4π + 2π) cycloaddition reaction between a conjugated diene and a dienophile forms six-membered rings in the presence of heat or light, which can be hydrogenated to cycloalkanes.