In the world of chemistry, molecules can be like twins. Sometimes they are perfect mirror images (Enantiomers), and sometimes they are related but not quite identical—kind of like siblings who share some features but don’t look exactly alike. That second group is where Diastereoisomers live.
What are Diastereoisomers?
Diastereoisomers (or diastereomers) are a type of stereoisomer. By definition, they are molecules that have the same chemical formula and the same connectivity of atoms, but they differ in how those atoms are arranged in space. They are not mirror images of each other and are not superimposable.
The “Chiral Center” Rule: To have diastereoisomers, a molecule usually needs at least two chiral centers (carbon atoms bonded to four different groups).
If a molecule has n chiral centers, the maximum number of stereoisomers is 2n.
- Enantiomers: Opposite configuration at all chiral centers. The configuration is swapped at every chiral center.
- Diastereoisomers: Opposite configuration at some, but not all, chiral centers.
Types of Diastereoisomerism
Diastereoisomerism isn’t just about chiral carbons; it shows up in two main ways:
A. Molecules with Multiple Chiral Centers
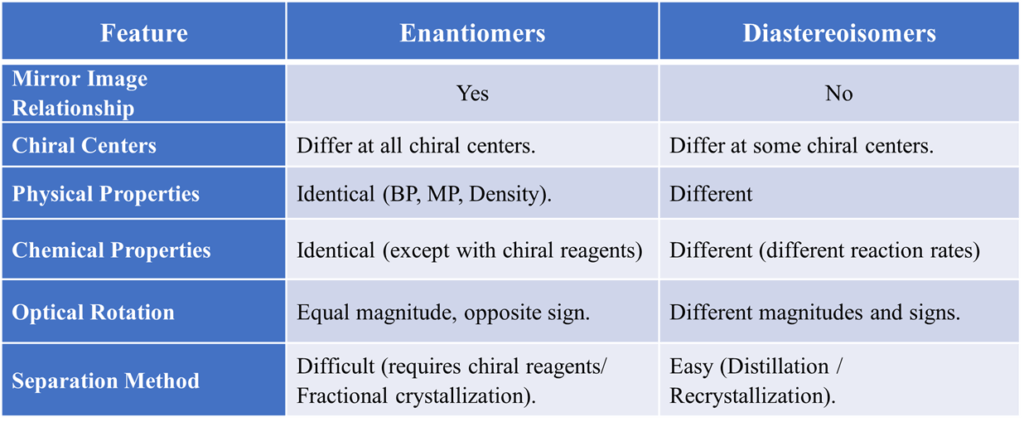
If a molecule has two chiral centers (C1 and C2), we label their spatial arrangement as either R or S. It can be better understood by tartaric acid.
- A molecule with (R, R) configuration and one with (S, S) are Enantiomers (complete opposites).
- A molecule with (R, R) configuration and one with (R, S) are Diastereoisomers (they match at one spot but differ at the other).
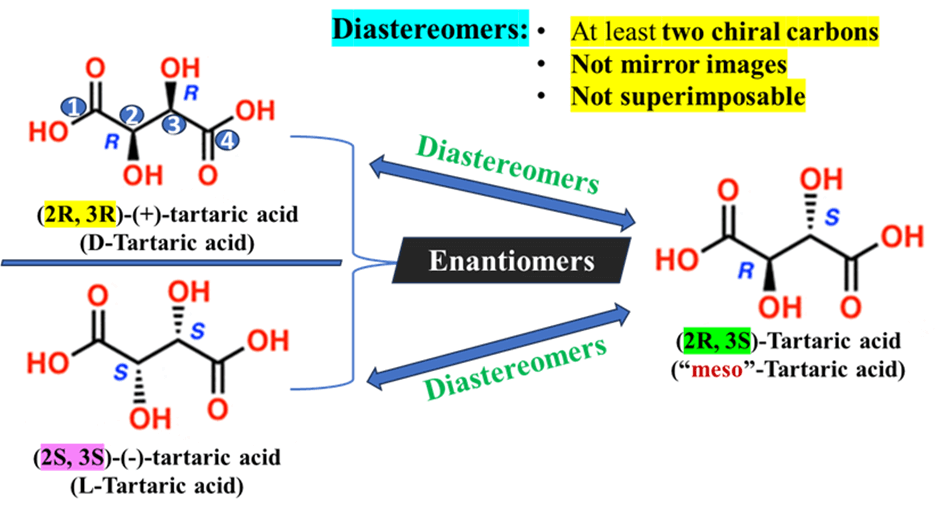
B. Geometrical Isomerism (Cis/Trans)
Diastereoisomerism isn’t limited to chiral centers; it also includes Cis-Trans (Geometric) Isomerism (like those found in alkenes). They have the same connectivity but are not mirror images of each other.
- Cis-2-butene: Identical Groups (the methyl groups) on the same side.
- Trans-2-butene: Identical Groups (the methyl groups) on opposite sides.
Physical and Chemical Properties
The most important part of the core concept is that spatial distance changes.
- In enantiomers, the distance between atoms is identical (like your two hands).
- In diastereomers, the distance between non-bonded atoms actually changes.
This is the most important practical difference between types of isomers:
- Physical Properties: Because the atoms are physically closer or further apart than in their sibling molecule, they bump into each other differently. This results in different physical properties like melting points, boiling points, solubility and densities.
- Separation: Because their physical properties differ, they can be easily separated by standard lab techniques like distillation, recrystallization, or chromatography.
- Chemical Reactivity: They react with the same reagents but often at different speeds because their shapes fit differently into reaction sites.
Summary in Simple Terms
Think of a pair of shoes.
- Your Left Shoe and Right Shoe are enantiomers (mirror images).
- Now, imagine your Left Shoe compared to your friend’s Left Shoe. They are both “left shoes,” but they aren’t mirror images of each other, and they aren’t exactly the same. That is diastereoisomerism.
In chemistry, this “partial difference” allows us to separate complex mixtures of drugs and chemicals effectively.
The Case of Tartaric Acid
Tartaric Acid is the classic textbook example because it perfectly illustrates how diastereoisomers and enantiomers coexist within the same set of molecules. Tartaric acid has two chiral centers. According to the 2n rule, we expect 4 isomers (22 = 4) due to presence of 2 chiral carbons, but there are actually only 3 because one form is a Meso compound.
The Enantiomeric Pair
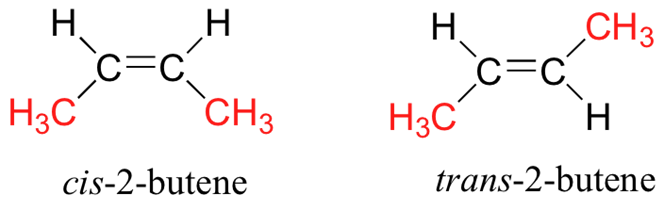
- (+)-Tartaric Acid: Rotates plane-polarized light to the right.
- (-)-Tartaric Acid: Rotates plane-polarized light to the left.
- Relationship: These two are mirror images of each other. They rotate plane-polarized light in equal but opposite directions (+12° and -12°). They have identical melting points and boiling points.
The Meso Form
Meso-Tartaric Acid: This version has an internal plane of symmetry (the top half is a mirror image of the bottom half). Even though it has chiral centers, the top half “cancels out” the bottom half, making the whole molecule achiral (optically inactive).

Relationship to the others: Meso-tartaric acid is a diastereoisomer of both the (+) and (-) forms. It is not a mirror image of them. It has completely different physical properties (like a different melting point).
Quick Reference of properties comparison is given below as below:
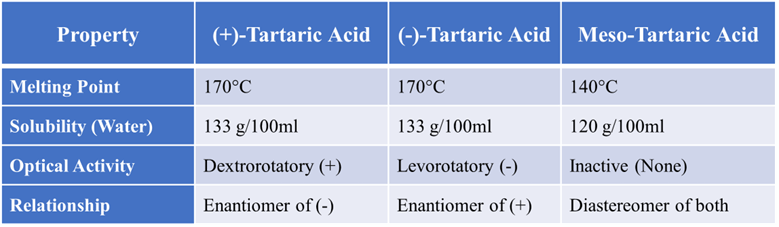
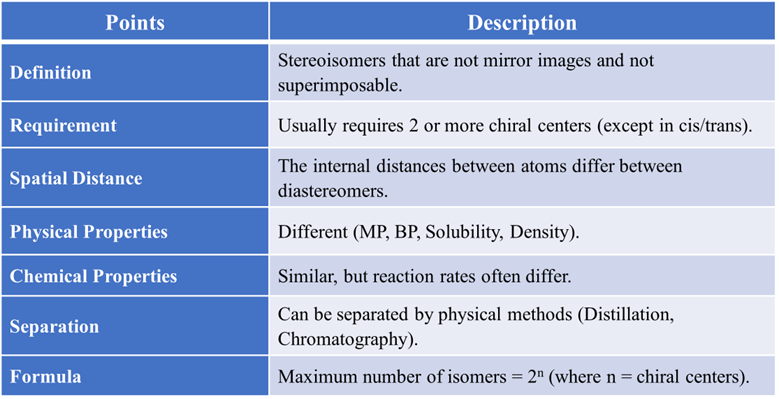
Summary Table of All Key Points
Why Diastereoisomers Matter in Real Life?
Pharmacology and Medicine
The human body is highly “chiral.” Our receptors, enzymes, and DNA are shaped in specific ways, meaning they only fit with specific molecular shapes—much like a lock only accepts one specific key. Because diastereoisomers have different shapes, often only one diastereoisomer of a drug fits the target receptor to provide a cure. The other might be completely inactive or bind to a different receptor, causing unintended side effects.
The Food and Flavor Industry
Our senses of taste and smell rely on protein receptors in the nose and tongue. Because diastereoisomers have different spatial arrangements, they “plug into” these receptors differently.
- Sweetness vs. Bitterness: For example, some diastereoisomers of artificial sweeteners like Aspartame taste sweet, while others can taste bitter or have no taste at all.
- Aromas: In essential oils, one diastereoisomer might smell like roses, while its “sibling” molecule might smell like wood or citrus.
Resolution (Separation) of Enantiomers
Enantiomers (mirror images) are notoriously hard to separate because they have the same boiling and melting points. Chemists solve this by reacting the mixture with a chiral “resolving agent.”
- The enantiomers turn into diastereoisomers.
- Because diastereoisomers have different solubilities, one will crystallize out of the liquid while the other stays dissolved.
- This allows to separate them easily using basic lab equipment and the production of pure, single-isomer drugs.
Synthetic Chemistry
When making complex molecules like vitamins or antibiotics, chemists use diastereoselective reactions. This means they choose specific catalysts or conditions to ensure the reaction builds the “correct” diastereoisomer needed for the final product.
Difference between Enantiomers and Diastereoisomers