The D/L system (also known as the Fischer-Rosanoff convention) is one of the oldest method used to designate the configuration of optical isomers. While the modern R/S system is more comprehensive for general chemistry, the D/L system remains the standard for describing amino acids and sugars. The D/L system is the “biological language.” It tells us about the lineage (parentage) and shape of the molecules that actually build life.
How to Assign D or L
To determine the configuration of a more complex molecule, you must first orient it as a Fischer projection according to these rules:
- Vertical Chain: Place the longest carbon chain vertically.
- Most Oxidized Carbon: Place the most highly oxidized carbon (like an aldehyde CHO or carboxylic acid COOH) at the top.
- The Bottom Chiral Center: Look at the chiral carbon most distant from the top carbonyl group (the “penultimate” carbon).
The Rule of Thumb:
- If the characteristic group (e.g. —OH in sugars, —NH₂ in amino acids) is on the right, it is the D-isomer (Dextro).
- If the characteristic group is on the left, it is the L-isomer (Levo).
Note: A “D” molecule can be either (+) or (-). The D/L label tells you how it looks, not how it behaves in a beam of light.
Common Applications:
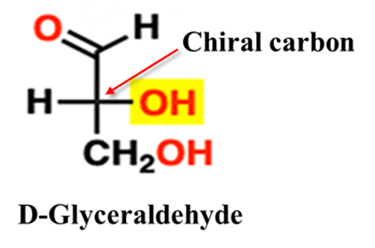
Glyceraldehyde (The Reference Standard)
The D/L system is based on the structure of glyceraldehyde, the simplest chiral sugar. Because it has only one chiral center, it serves as the “ruler” for all other molecules.
- D-Glyceraldehyde: The hydroxyl group (—OH) is on the right side in a Fischer projection.
- L-Glyceraldehyde: The hydroxyl group (—OH) is on the left side in a Fischer projection.
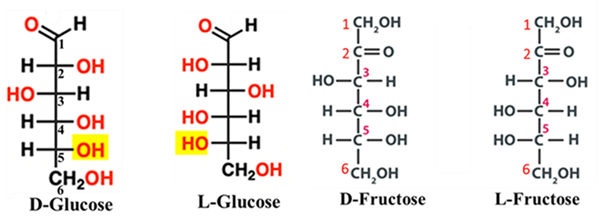
Sugars (Carbohydrates)
In glucose, for example, we look at the lowest chiral center at Carbon-5. In nature, almost all significant sugars have the hydroxyl group (—OH) on the right side in a Fischer projection. These are D-sugars.
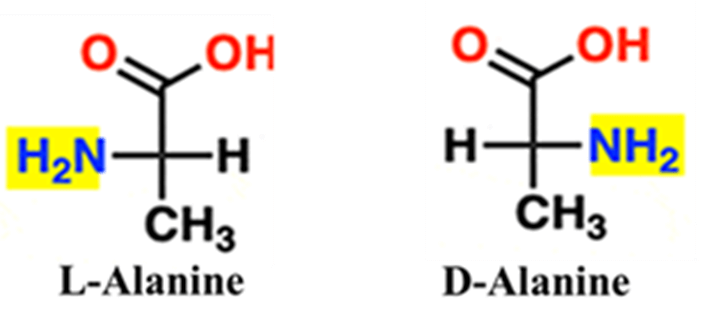
Amino Acids
For amino acids, we look at the position of the amino group (—NH2). Interestingly, while sugars are mostly D-form, almost all naturally occurring protein-building amino acids are L-amino acids.
Conversion of a 3D wedge-and-dash structure into a Fischer projection
To get the D/L label right, you have to convert a 3D structure into a Fischer projection. It is like “flattening” a molecule against a wall. You have to look at the molecule from a specific perspective.
Here is the step-by-step process to ensure you don’t get the orientation flipped:
The “Bow-tie” view
Imagine the chiral carbon is the center of a bow-tie.
- The horizontal bonds must be pointing toward you (wedges).
- The vertical bonds must be pointing away from you (dashes).
To convert a standard 3D wedge-dash drawing, rotate the molecule in your mind until the carbon chain is vertical and curving away from you, like a spine.
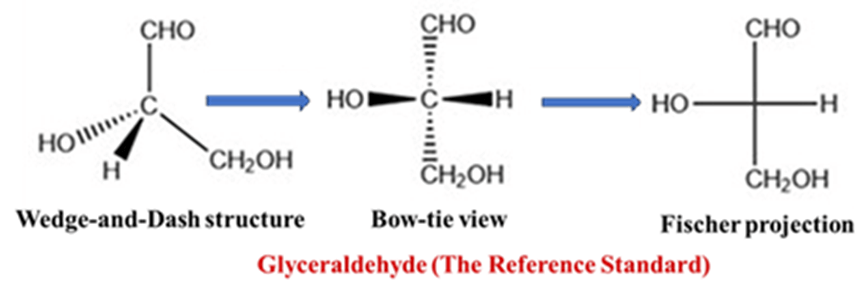
Drawing the Projection
Once you have the correct orientation, simply “squash” it onto the paper:
- The chiral carbon becomes a crosshair (+).
- The groups on the horizontal bonds (wedges) go to the left and right.
- The groups on the vertical bonds (dashes) go to the top and bottom.
Applying the D/L Test
Now that it’s flat, apply the rules we discussed. Let’s use sugars and amino acids as the primary examples:
For Sugars (The —OH Rule): Check the —OH group on the chiral carbon furthest from the aldehyde/ketone group.
For Amino Acids (The —NH₂ Rule): Follow the exact same logic, but you look for the -NH2 group position instead of the -OH group. Remember to keep the -COOH group at the top.
Why does this matter?
Almost every protein in your body is made exclusively of L-amino acids (“L” Proteins). The backbone of your DNA (deoxyribose) and the sugar in your blood (glucose) are D-sugars. If you were to eat “Mirror-Image Food” (D-amino acids and L-sugars), your enzymes wouldn’t recognize them. You could eat a full meal of “L-glucose” and starve to death with a full stomach because your body lacks the “key” to unlock that specific molecular “lock.”
Low-Calorie Sugars
Some “artificial” sweeteners are actually just L-sugars (like L-fructose). They taste sweet because they hit the tongue’s receptors, but because they are the “wrong” D/L configuration, your body can’t digest them, resulting in zero calories.
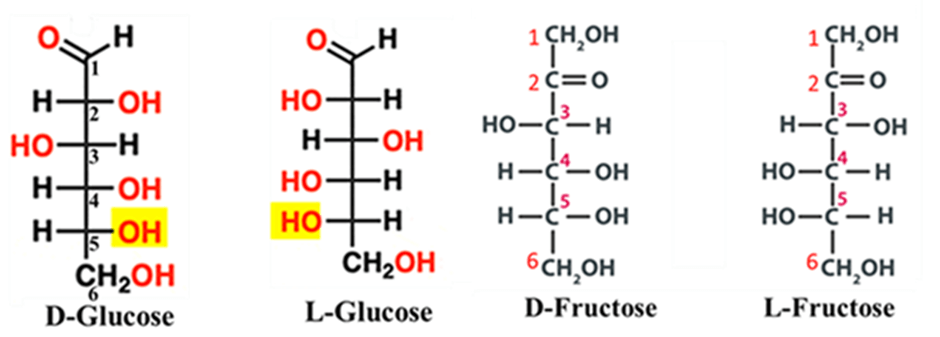
The “Invert” Sugar
In the food industry, you may hear about Invert Sugar (found in honey or candy making). Invert sugar is a liquid sweetener made by splitting sucrose (table sugar) into equal parts glucose and fructose through hydrolysis. Sucrose is dextrorotatory (+). When hydrolyzed (broken down), it turns into a mixture of D-Glucose and D-Fructose. Even though they are both D-isomers (based on their structure), the Levo-rotation of Fructose is so strong that the whole mixture now rotates light to the left (-).
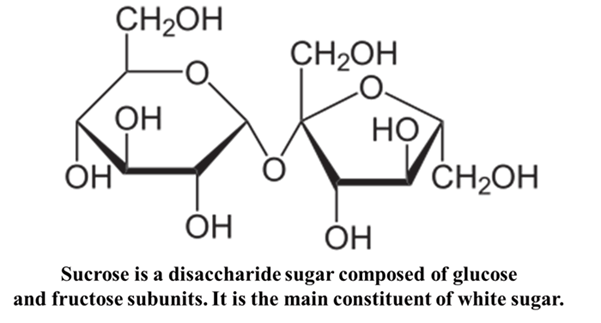
This is why we must be careful: The D/L tells us the genetic “shape” of the sugar, but it doesn’t always tell us which way the light will bend.
Why we still use D/L today ?
You might wonder: If R/S is more accurate, why do we keep D/L?
Naming a complex sugar like Glucose using the R/S system is a bit like giving someone GPS coordinates for every single turn in a journey—it’s precise, but exhausting. You’d have to call it (2R, 3S, 4R, 5R)-2,3,4,5,6-pentahydroxyhexanal.
In contrast, the D/L system acts like a family name. Here is why we still use it for sugars:
The “Family” Shortcut
Instead of labeling every single carbon, the D/L system only cares about the penultimate carbon (the one at the very bottom of the chain, Carbon-5 in glucose).
- If that one carbon matches D-Glyceraldehyde, the whole molecule is labeled “D“.
- This single letter tells a biologist that the sugar is “right-handed” in its overall architecture and is likely digestible by human enzymes.
Structural Consistency
In a Fischer projection, all D-aldoses (like D-Glucose, D-Galactose, and D-Mannose) have their bottom-most hydroxyl group on the right.
Biological Recognition
Your body is “chiral-selective”. It’s built to recognize the D-family of sugars. Using “D-Glucose” is more practical for a doctor or nutritionist than the R/S coordinates because:
- D-Glucose is blood sugar (fuel).
- L-Glucose is a synthetic mirror image that tastes sweet but cannot be used by your cells for energy.
Summary: Why we use D/L for sugars
- R/S = The “Social Security Number” (unique, precise, but complex).
- D/L = The “Last Name” (identifies the family and biological behavior instantly).
Key Conceptual Differences
While both systems describe the 3D arrangement (configuration) of atoms in a molecule, they serve different purposes and use entirely different rules.
The D/L System (Visual)
You orient the molecule vertically with the most oxidized carbon at the top. You only look at the bottom-most chiral center.
- D: Functional group is on the Right.
- L: Functional group is on the Left.
The R/S System (Priority)
You assign a priority (1 to 4) to each group attached to the chiral center based on Atomic Number. You then look down the bond of the lowest priority group (#4).
- R (Rectus): Sequence 1 → 2 → 3 is Clockwise.
- S (Sinister): Sequence 1 → 2 → 3 is Counter-clockwise.
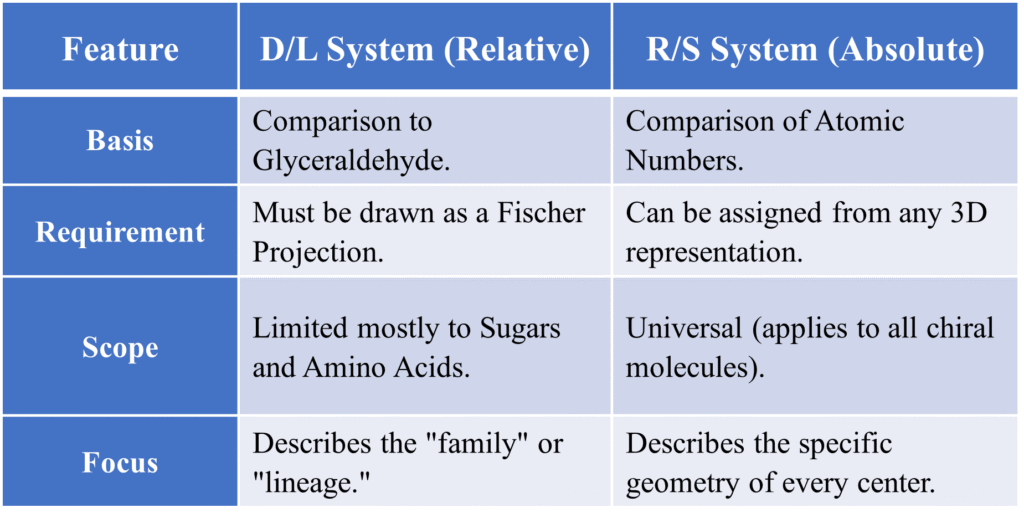
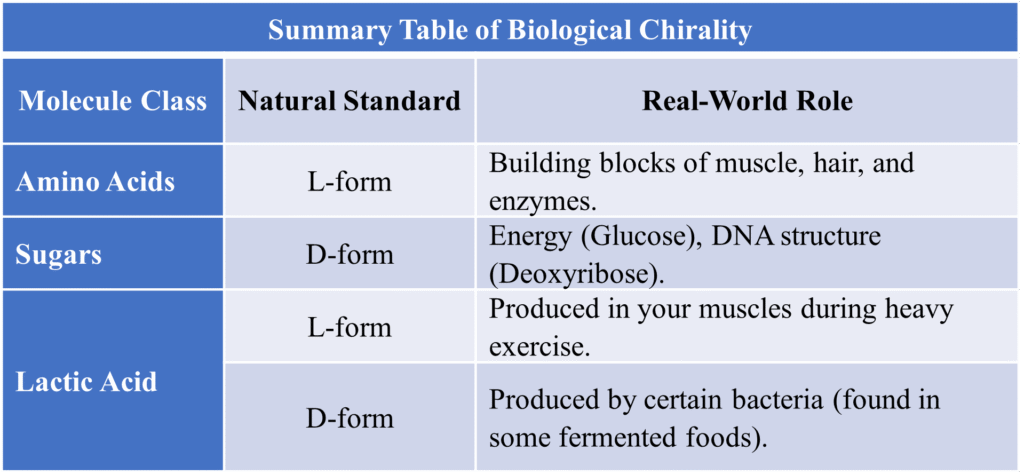
Summary Table of Biological Chirality