In Pharmaceutical Chemistry, Enantiomerism is vital because the human body is a chiral environment composed of chiral building blocks like L-amino acids and D-sugars. Consequently, the body treats mirror-image drug molecules very differently.
Definition
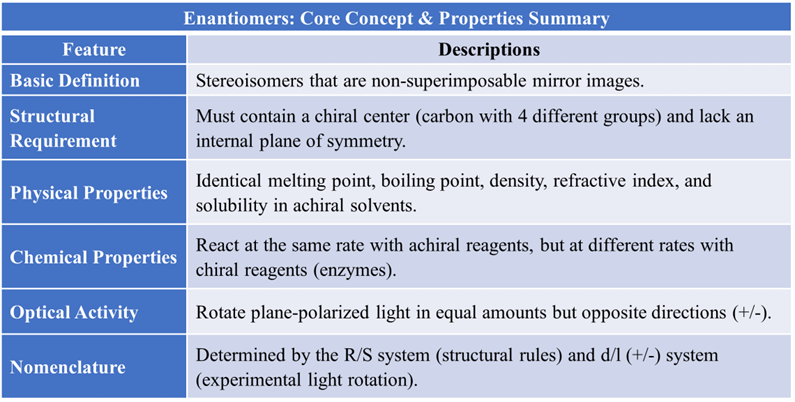
Enantiomerism is a type of optical isomerism where molecules lack an internal plane of symmetry. It is vital concept because most biological targets (receptors, enzymes, and DNA) are chiral, meaning they can distinguish between two mirror-image drug molecules.
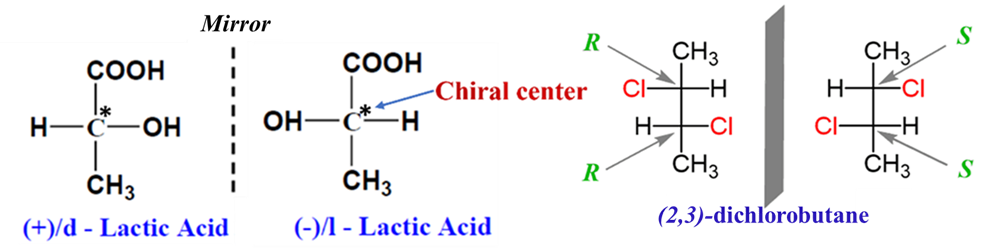
Enantiomers are a pair of stereoisomers that are non-superimposable mirror images of each other. This relationship is similar to your left and right hands; they look identical in a mirror but cannot be perfectly covered on top of each other.
Core Characteristics
- Chirality: Enantiomerism typically occurs in molecules containing a chiral center, which is a carbon atom bonded to four different groups. These molecules lacking an internal plane of symmetry.
- Mirror Image Relationship: They are related like a person’s left and right hands; they look identical in a mirror but cannot be perfectly stacked on top of one another.
- Biological Context: The human body is a chiral environment made of chiral building blocks like L-amino acids and D-sugars. Because of this, the body treats mirror-image drug molecules (enantiomers) very differently.
- Optical Activity: Enantiomers have the ability to rotate the plane of Plane Polarized Light (PPL).
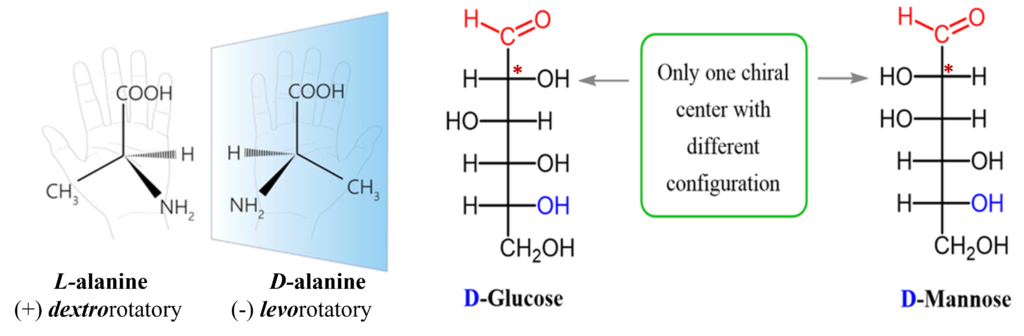
- Dextrorotatory (+): Rotates light to the right (clockwise).
- Levorotatory (-): Rotates light to the left (counter-clockwise).
- This phenomenon is a cornerstone of stereochemistry and is vital for identifying drugs and checking their purity in pharmaceutical analysis.
Nomenclature and Identification
- Absolute Configuration: Enantiomers are labeled using the R and S system based on structural rules to describe the absolute configuration of chirality centers.
- Optical Rotation: They are also identified by their direction of light rotation: d (+) for dextrorotatory (clockwise/right) or l (-) for levorotatory (anti-clockwise/left).
- Distinction: There is no relationship between the R/S configuration and the d/l (+/-) notations; R/S is determined by structural rules, while (+/-) is determined only by experiment.
Properties of Enantiomers
Enantiomers behave identically in a symmetric environment but differently in an asymmetric (chiral) environment. Enantiomers have unique physical and chemical characteristics that are essential for pharmaceutical analysis:
Physical Properties
In standard laboratory conditions, enantiomers are like identical twins. They are very difficult to separate because most of their physical constants are the same.
- Identical Constants: They have the exact same melting point, boiling point, density, and refractive index.
- Solubility: They have the same solubility in ordinary (achiral) solvents like water, ethanol, or benzene.
- Spectroscopy: Their UV-Visible, IR, and NMR spectra are identical.
- Optical Activity (The Critical Difference): This is the only physical way to tell them apart. Enantiomers rotate plane-polarized light in equal amounts but in opposite directions.
Chemical Properties
Their reactivity depends entirely on what they are reacting with.
- Reaction with Achiral Reagents: If the reagent is not chiral (e.g., HCl or NaOH), both enantiomers react at the same rate.
- Reaction with Chiral Reagents: If the reagent is chiral (like a specific enzyme or a chiral catalyst), the two enantiomers react at different rates. This is the basis for Chiral Resolution—the process of separating a racemic mixture into its pure components.
Biological Properties
Because the human body is made of chiral building blocks (L-amino acids and D-sugars), it acts as a chiral environment. Biological Properties includes binding affinity, pharmacokinetics (ADME) studies, toxicology etc. of drug molecules.
The Racemic Mixture
A Racemic Mixture (or racemic modification) is a 50:50 mixture of two enantiomers.
- Optical Inactivity: Because the (+) rotation of one enantiomer exactly cancels out the (-) rotation of the other, a racemic mixture has zero optical activity.
- Pharmacy Context: Many synthetic drugs are sold as racemic mixtures (like Ibuprofen), even if only one isomer is active. (S)-Ibuprofen is the active isomer that reduces pain and inflammation while (R)-Ibuprofen is the “inactive” isomer.
Identification and evaluation:
In pharmaceutical sciences, the identification and evaluation of enantiomers are critical because the human body is a “chiral environment” that treats mirror-image molecules differently. Identification ensures the correct isomer is present, while evaluation determines the purity and stability of the drug.
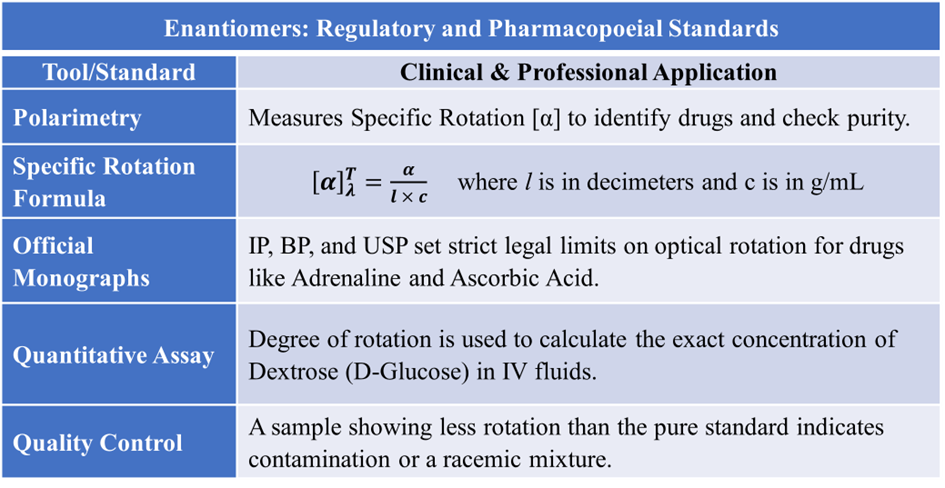
A. Polarimetry (Identification by Optical Activity)
The primary method for identifying enantiomers is measuring their Optical Activity. Enantiomers rotate Plane Polarized Light (PPL) in opposite directions. Every optically active drug has a unique Specific Rotation [α] value used as a “fingerprint”. For example, Adrenaline must be the levorotatory (-) form; a (+) rotation indicates the wrong isomer. Evaluation is performed using the Specific Rotation formula.
B. Evaluation of Optical Purity (Enantiomeric Excess)
Pharmacists evaluate how “pure” a chiral drug is by comparing its observed rotation to a standard. A 50:50 Racemic Mixture of (+) and (-) enantiomers will show zero optical activity due to external compensation. If a sample (like (S)-Ibuprofen) shows less rotation than the pure standard, it indicates contamination by the other enantiomer.
C. Stability and Racemization Testing
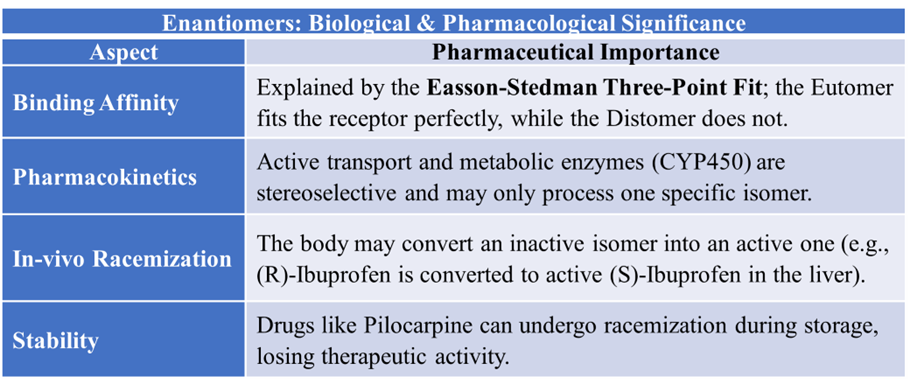
Evaluation includes monitoring if a drug undergoes Racemization over time. This occurs when a pure enantiomer converts into an inactive mixture due to heat, light, or pH changes. By measuring optical activity during stability testing, pharmacists determine the expiration date or Shelf-life (e.g., for Pilocarpine).
D. Quantitative Analysis (Assay)
For clear solutions, the degree of rotation is used to calculate the exact concentration of the drug. The concentration of dextrose in intravenous (IV) fluids is routinely checked using polarimetry.
Pharmacological Significance in the Human Body
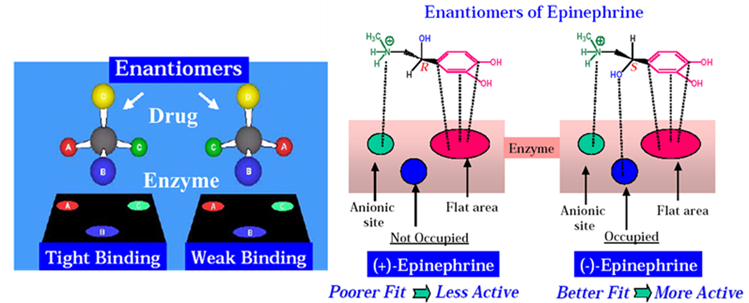
In pharmaceutical sciences, the interaction between a drug and its receptor is often described using the “Lock and Key” hypothesis. Because the “machinery” of the body is asymmetric, it treats enantiomers as two distinct chemical species. The receptors in the human body are made of chiral building blocks like L-amino acids (in proteins/enzymes) and D-sugars (in DNA/RNA), they act as chiral “locks” that can distinguish between the different 3D shapes of enantiomeric “keys” (isomers). For a drug to produce an effect, it must bind to a receptor. The Easson-Stedman Hypothesis proposes that for a drug to be effective, it must have at least three points of contact with its receptor. These sites are usually represented as: Hydrogen bonding sites, Hydrophobic pockets and Ionic interaction sites.
Difference in Pharmacological Activity (Eutomer vs. Distomer)
Imagine a receptor surface with three complementary binding “pockets” labeled A’, B’, and C’. Now, look at a chiral drug with functional groups A, B, and C.
The Eutomer (Active Isomer)
- The groups are arranged such that A binds to A’, B binds to B’, and C binds to C’.
- This creates a high-affinity “perfect fit,” leading to a strong pharmacological effect.
The Distomer (Less Active/Inactive Isomer)
- Because the groups are mirrored, when the distomer approaches the receptor, it can only align two groups (e.g., A and B).
- The third group (C) is pointing in the wrong direction and cannot bind to C’.
- This results in a “weak fit” or no fit at all, leading to lower potency or total inactivity.
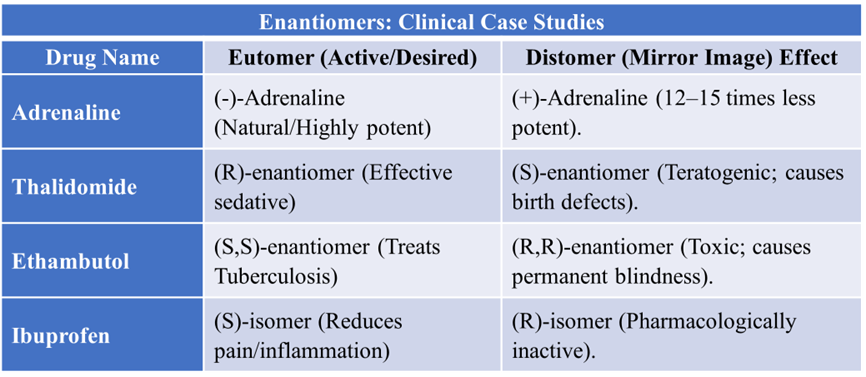
Classic Example: Adrenaline (Epinephrine)
- Structure: Adrenaline has a chiral carbon with three key groups: a Hydroxyl (-OH) group, a Catechol ring, and an Amine group.
- Binding: The adrenergic receptor has three matching binding sites for these groups.
- The Result: * (-)-Adrenaline (Natural): All three groups line up perfectly. It is highly potent. (+)-Adrenaline (Synthetic mirror): Only two groups (the ring and the amine) can bind. The -OH group points away. Consequently, it is about 12 to 15 times less potent than the (-) form.
Toxicological Significance
In some cases, the distomer is not just “inactive” but carries dangerous side effects.
- Thalidomide: While the (R)-enantiomer acts as a sedative, the (S)-enantiomer is teratogenic, leading to severe limb deformities in newborns.
- Ethambutol: The (S,S)-isomer is a powerful anti-tuberculosis agent, but the (R,R)-isomer can cause permanent optic nerve damage (blindness).
Metabolic Stability and Racemization
The body can sometimes change the stereochemistry of a drug after it is swallowed.
- In-vivo Racemization: Some drugs undergo a process where a pure, active enantiomer converts into an inactive mixture due to the body’s pH or heat. Example: Ibuprofen. The inactive (R)-isomer is converted by enzymes in the liver into the active (S)-isomer.
- Pilocarpine: Used for glaucoma, it can lose its therapeutic activity if it undergoes racemization during storage or within the body.
Regulatory Significance of Enantiomerism
Regulatory Requirements for Drug Standards
In the pharmaceutical industry, enantiomeric purity is not just a chemical preference; it is a legal requirement. Regulatory bodies (like the FDA and CDSCO) mandate that for any chiral drug, the manufacturer must prove the safety and efficacy of the specific isomer being used. Pharmacists and Quality Control (QC) analysts use Specific Optical Rotation as a molecular “fingerprint.” Every pure enantiomer has a unique specific rotation value. This is used to verify the identity of raw materials during the procurement stage.
Pharmacopoeial Standards (IP, BP, USP)
Official monographs in the Indian Pharmacopoeia (IP), British Pharmacopoeia (BP), and United States Pharmacopeia (USP) set strict limits on optical activity for chiral substances. If a drug is known to be optically active (e.g., Adrenaline, Atropine, or Ascorbic Acid), its specific rotation range must be documented and tested. A batch of medicine is considered “Substandard” if it fails to meet the specific rotation range defined in its official monograph.
Quantitative Analysis: Assay and Concentration
Enantiomerism is used as a tool for Quantitative Analysis (measuring how much of a drug is present). This is particularly vital in hospital pharmacy for large-volume parenterals. Dextrose (D-Glucose) is the D-enantiomer of glucose. In IV fluids, the concentration must be precise (e.g., 5% , 10%, or 25%). By measuring the observed rotation (α) of the solution, concentration is calculated by following formula:
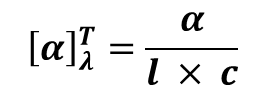
Stability and Storage (Racemization)
Enantiomers are not always stable. Over time, or due to heat/pH changes, a pure enantiomer may slowly convert into its mirror image, forming a racemic mixture. If a drug racemizes in the bottle, its potency drops by 50% or more, and it may become toxic. Monitoring optical rotation is a standard part of Stability Testing and determining Expiry Dates.
Summary
Core Concept & Properties Summary
Biological & Pharmacological Significance
Clinical Case Studies
Regulatory and Pharmacopoeial Standards