Introduction
Naphthalene is a simple organic compound made of two benzene rings fused together, looking a bit like a double hexagon. In its physical form, it appears as shiny, white crystals or flakes. Naphthalene is famous for sublimation. This means it turns directly from a solid crystal into a gas without ever melting into a liquid. This gas is what creates a protective “shield” around clothes to keep moths away.
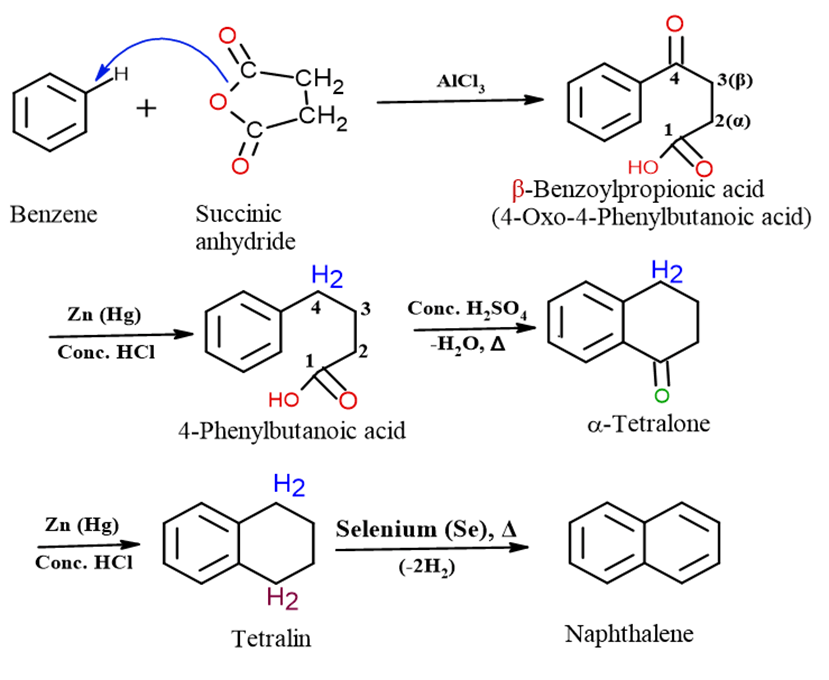
The Haworth Synthesis is a multi-step process used for the preparation of linear polynuclear aromatic hydrocarbons, most commonly Naphthalene and Anthracene. It involves the addition of a four-carbon chain to a Benzene ring, followed by cyclization and aromatization.
The synthesis of Naphthalene proceeds through the following four stages:
Step 1: Friedel-Crafts Acylation
Benzene is reacted with succinic anhydride in the presence of a Lewis acid catalyst, such as anhydrous AlCl3. This electrophilic substitution reaction adds the anhydride ring to the benzene, opening it up to form β-Benzoylpropanoic acid.

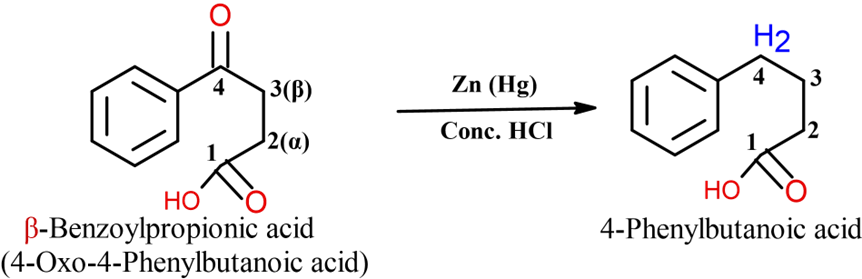
Step 2: Clemmensen Reduction
The carbonyl group (C=O) in the side chain of 3-benzoylpropanoic acid is then reduced to a methylene group (CH2) using Clemmensen reduction (Zinc amalgam (Zn/Hg) in concentrated HCl or Wolf-Kishner reduction. This yields 4-phenylbutanoic acid.
Step 3: Intramolecular Friedel-Crafts Acylation (Cyclization)
4-Phenylbutanoic acid is treated with a dehydrating and cyclizing agent, typically concentrated H2SO4 or HF. The ring-closure occurs via an intramolecular Friedel-Crafts acylation, forming the six-membered ring structure, alpha-tetralone (1-oxo-1,2,3,4-tetrahydronaphthalene).

Step 4: Reduction and Dehydrogenation (Aromatization)
1-Reduction: The remaining carbonyl group in alpha-tetralone is reduced using Clemmensen reduction to give Tetralin (1,2,3,4-tetrahydronaphthalene).

2- Dehydrogenation (Aromatization): Finally, Tetralin is heated with a catalyst like Sulfur (S), Selenium (Se), or Palladium on Charcoal (Pd/C) at a high temperature. This removes four hydrogen atoms (dehydrogenation), converting the cyclohexene ring into an aromatic ring, yielding the final product, Naphthalene.

Summary: Multi-step Haworth Synthesis of Naphthalene