Limit tests are quantitative or semi-quantitative tests designed to identify and control small quantities of impurities which are likely to be present in a substance. Think of these as “pass/fail” tests to ensure chemical purity. It determines if the impurity is below the maximum allowable limit specified by the Pharmacopoeia.
Definition & Logic
- Definition: Semi-quantitative tests to detect and limit impurities in pharmaceuticals.
- The Rule: Test (T) ≤ Standard (S) → PASS (Complies). To pass a test, the “Test” solution must be less intense (clearer, lighter, or less stained) than the “Standard.”
- The Vessel: Nessler Cylinder (for Chloride, Sulphate, Iron)-
- It is a long, narrow glass tube with a flat bottom and 50 mL capacity. It is used in a pair (one for ‘Test’ and one for ‘Standard’) to ensure the comparison is fair.
- Uniformity: Both cylinders have the same diameter and glass thickness so that the path of light is identical.
- Observation: For turbidity/opalescence- always view them transversely (from the side) against a black background; For color view vertically (looking down) against a white background.
- Gutzeit Apparatus (for Arsenic): It is a specialized wide-mouthed glass bottle (120 mL) fitted with a rubber stopper and a glass tube.
- Caution: Always use distilled water for all steps.

Limit Test for Chloride
Principal and reaction
The limit test for chloride is based on the chemical reaction between soluble chloride ions and Silver Nitrate in the presence of dilute nitric acid. The reaction produces Silver Chloride (AgCl), which is insoluble in dilute nitric acid and appears as a milky cloudiness called opalescence.
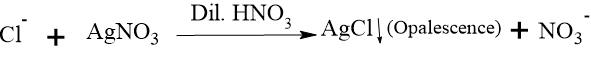
Role of Nitric Acid: It is added to make the solution acidic and to prevent the precipitation of other acid radicals (like carbonates or phosphates) which might interfere with the test.
Procedure
Prepare two Nessler cylinders and label them as “Test” and “Standard“.

Observation
The opalescence of the “Test” solution is compared with a “Standard” solution. It is done by viewing them transversely (from the side) against a black background.
- Standard Solution: Shows a slight white opalescence.
- Test Solution: The opalescence is [Less than / More than] that of the standard.
Inference (Result)
- If Test < Standard: The opalescence produced in the test solution is less than the standard solution. Therefore, the given sample passes the limit test for chloride and is of pharmacopoeial grade.
- If Test > Standard: The opalescence produced in the test solution is more than the standard solution. Therefore, the given sample fails the limit test for chloride.
Limit Test for Sulphate
Principal and reaction
The limit test for sulphate is based on the reaction of soluble sulphate with Barium Chloride in the presence of dilute hydrochloric acid to form Barium Sulphate. Barium sulphate is insoluble in dilute HCl and appears as a white cloudiness known as turbidity.

- Role of Hydrochloric Acid: It makes the solution acidic and prevents the precipitation of other ions (like oxalates or phosphates) that might otherwise react with barium.
- Role of Barium Sulphate Reagent: This is a specialized reagent. It contains barium chloride, sulfate-free alcohol (to prevent supersaturation), and a small amount of potassium sulphate (to increase sensitivity of the test).
Procedure
Prepare two Nessler cylinders and label them as “Test” and “Standard“.
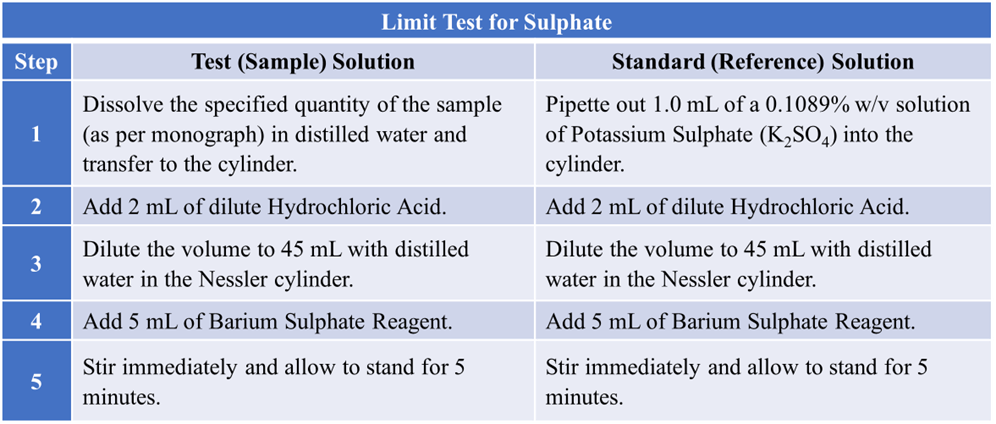
Observation
Compare the turbidity of both solutions by looking through the side of the cylinders against a black background.
- Standard Solution: Shows a distinct white turbidity.
- Test Solution: The turbidity is [Less than / More than] that of the standard solution.
Inference (Result)
- Option 1 (Pass): The turbidity produced in the test solution is less than that of the standard solution. Therefore, the given sample complies with the limit test for sulphate as per IP and is of pharmacopoeial quality.
- Option 2 (Fail): The turbidity produced in the test solution is more than that of the standard solution. Therefore, the given sample does not comply with the limit test for sulphate.
Limit Test for Iron
Principal and reaction
This test is based on the reaction of iron with thioglycolic acid in a medium made alkaline with ammonia. This produces a pale pink to deep reddish-purple color complex due to the formation of ferrous thioglycolate.
Reduction: Thioglycolic acid reduces any Ferric (Fe3+) iron present in the sample to Ferrous (Fe2+) iron.
Complexation:
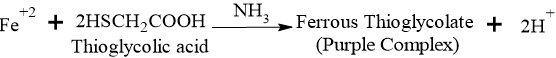
- Role of Citric Acid: Iron tends to precipitate as Iron Hydroxide when ammonia is added. Citric acid forms a soluble complex with iron, keeping it in solution so it can react with the thioglycolic acid.
- Role of Ammonia: The purple color is only stable and visible in an alkaline medium.
Procedure
Prepare two Nessler cylinders and label them as “Test” and “Standard“.

Observation
Compare the intensity of the purple color by viewing the cylinders vertically (looking down from the top) against a white background.
- Standard Solution: A pale purple/pink color is produced.
- Test Solution: The intensity of the purple color is [Less than / More than] that of the standard.
Inference (Result)
- Pass: The color intensity of the test solution is less than the standard solution. Therefore, the sample complies with the limit test for Iron as per IP.
- Fail: The color intensity of the test solution is more than the standard solution. Therefore, the sample does not comply with the limit test for Iron.
Limit Test for Arsenic
Principal and reaction
It is also known as the Gutzeit Test. Arsenic in the sample is reduced to arsenious acid, then to Arsine gas (AsH3) by nascent hydrogen generated from zinc and HCl. This arsine gas travels up the tube and reacts with mercuric chloride paper to form a yellow or brown stain.
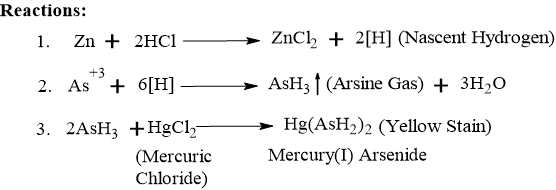
- Stannated Hydrochloric Acid: It is a specific reagent where a small amount of Stannous Chloride (SnCl2) is added to concentrated HCl. The “Stannous” part of the acid acts as a reducing agent. It converts all the pentavalent arsenic (As5+) in the sample into the trivalent (As3+) state, which reacts much faster and more completely.
- Zinc: Stannous chloride reacts with the zinc to form a Zinc-Tin couple (a coating of tin on the zinc). This “couple” acts as a catalyst, ensuring a steady and continuous stream of hydrogen gas for the full 40 minutes of the test.
- Role of Lead Acetate Cotton: Hydrogen Sulphide (H2S) is a common impurity that can also stain the paper black. The lead acetate cotton traps H2S as Lead Sulphide, ensuring only Arsenic reacts with the paper.
Procedure
Arrange two Gutzeit Apparatus and label them as “Test” and “Standard“. Always check the bottle label for the words “AsT” (Arsenic Test grade) or “Arsenic-Free.”

- Why 40 minutes? The reaction is slow; it takes time for all the arsenic to be converted to gas and travel up the tube.
- Why warm? Gentle heat (approx. 40°C) helps maintain a steady evolution of gas.
Observation
Compare the yellow stain produced on the mercuric chloride paper of both the Test and the Standard.
- Standard: A distinct yellow/brown stain is observed.
- Test: The intensity/depth of the stain is [Lighter / Darker] than the standard.
Inference (Result)
- Pass: The yellow stain produced by the test sample is less intense than that of the standard. The sample complies with the limit test for Arsenic.
- Fail: The yellow stain produced by the test sample is more intense than that of the standard. The sample does not comply with the limit test for Arsenic.