Understanding stereochemistry isn’t just about passing an exam. It’s about understanding how drugs interact with the body. Some molecules are like “chemical illusions,” and today we’re talking about a classic one: the Meso Compounds.
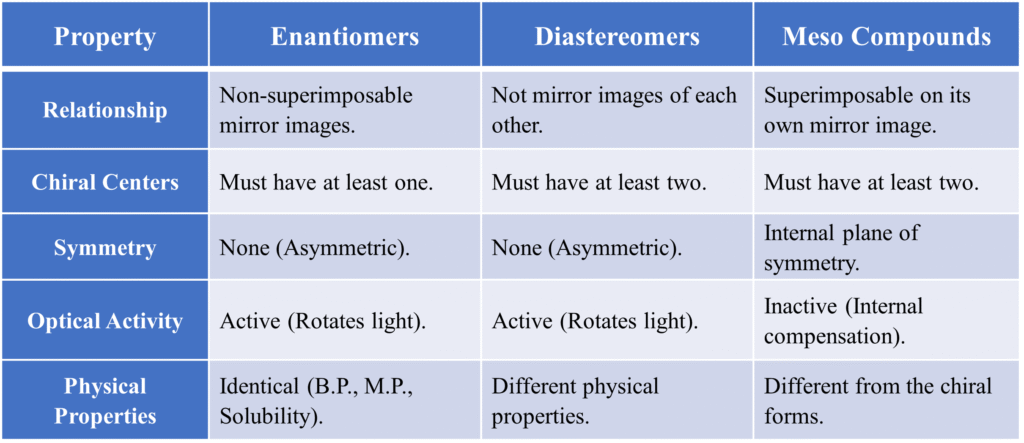
What is a Meso Compound?
By definition, a meso compound is a molecule that contains tetrahedral stereocenters (usually two or more chiral / asymmetric carbons) but is overall optically inactive due to an internal plane of symmetry.
Wait—how can a molecule have “chiral” parts but not be chiral itself? It’s because of internal compensation.
The Key Ingredients
To be a meso compound, a molecule needs two things:
- Two or more chiral centers.
- An internal plane of symmetry (it can be cut into two identical halves).
Why are they Optically Inactive?
Normally, chiral centers rotate plane-polarized light. However, in a meso compound:
- The top half of the molecule rotates light in one direction (e.g., clockwise).
- The bottom half rotates light in the exact opposite direction (counter-clockwise) by the same amount.
In a polarimeter, the optical rotation caused by one chiral center is exactly cancelled out by the equal and opposite rotation of the other half. This phenomenon is known as internal compensation. The net rotation is zero. Because they are superimposable on their own mirror images, meso compounds do not have enantiomers and behave as single, achiral substances.
How to Spot a Meso Compound?
To spot a meso compound in a drug structure, you just need to perform a quick “Symmetry Scan”. When you are looking at a drug structure or a Fischer projection, follow these steps:
- Check for “Chiral Centers”: Does it have at least two carbons attached to four different groups? If no, it’s just a regular achiral molecule.
- Check the “Ends”: Look at the top and bottom (or left and right) of the molecule. Are the terminal groups identical? (e.g., both are -COOH or both are -CH3).
- Look for the “Mirror Line”: Imagine a line cutting the molecule in half. If the top half reflects the bottom half exactly (like a butterfly’s wings), it is a Meso Compound.
Examples of meso compound
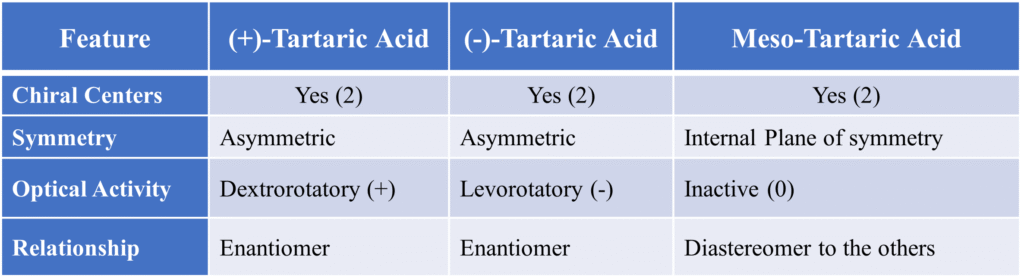
Meso-Tartaric Acid (2,3-dihydroxybutanedioic acid)
This is the classic, most frequently cited example. It is the poster child for meso compounds. It has two chiral centers (C2 and C3). There is a plane of symmetry dividing the molecule into two mirror-image halves (HOOC−𝐶𝐻(𝑂𝐻) on top and 𝐶𝐻(𝑂𝐻)−𝐶𝑂𝑂𝐻 on bottom). The top half is (𝑅) and the bottom half is (S), resulting in internal compensation (no net rotation). Hence optically inactive.
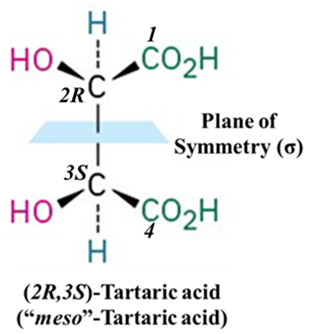
Let’s look at the difference between the enantiomers and the meso form:
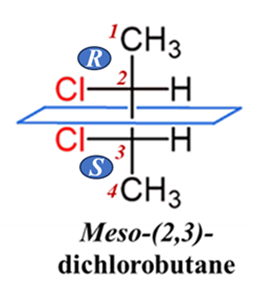
Meso-(2,3)-dichlorobutane
There are three Fischer projections. One of these is a meso compound, and the other two are enantiomers (a chiral pair).
Step-by-Step Analysis:
- Look at the side groups: In one of the structures, if you draw a dashed line through the center (between Carbon 2 and Carbon 3), the top half is a perfect mirror image of the bottom half.
- Check the rotation: In the chiral versions, both carbons might rotate light clockwise (R, R). In the meso version, one center is (R) and the other is (S).
- The Result: The (R) rotation cancels out the (S) rotation.
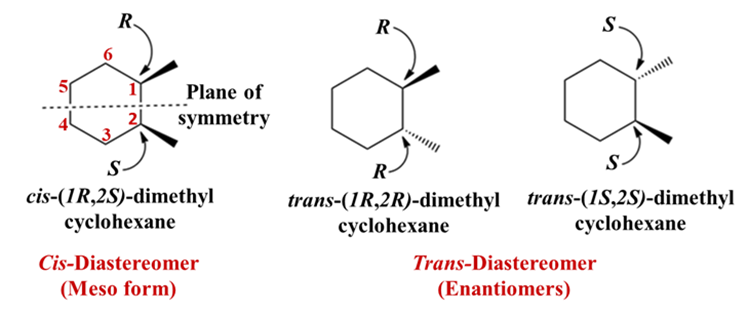
cis-1,2-dimethylcyclohexane
Even though it is a ring, you can draw a plane of symmetry right through the middle of the C1-C2 bond and the C4-C5 bond. It divides the molecule into two mirror images. The two chiral centers cancel each other out. In a “cis” configuration this is a meso compound.
Ethambutol
It is a common anti-tuberculosis medication.
- The (S,S)-enantiomer is the one that effectively treats TB.
- The (R,R)-enantiomer can actually cause blindness (optic neuritis).
- The Meso-form (R,S)? It is significantly less active and generally not used.
Summary for your Notes
- Meso = Chiral Centers + Internal plane of symmetry.
- They are superimposable on their mirror image.
- They do not have an enantiomer (the mirror image is just the same molecule again).
- The number of stereoisomers for a molecule with n chiral centers is usually 2n, but if meso forms exist, that total number decreases.
- Always look for the mirror! If you can fold the molecule in half and the groups match up perfectly, you’re looking at a Meso compound.
Note for Pharmacists
As a pharmacist, you need to know that just because a molecule has the “right” atoms (same molecular formula), if it’s in the meso configuration, it may be therapeutically useless or even toxic compared to its chiral counterparts. For example, some meso-isomers might be metabolized faster or bind differently to a receptor because of their specific shape.
The Stereoisomer Comparison Table