It is the standard nomenclature used to describe the absolute configuration of a chiral center (usually a carbon atom) in a molecule. To talk about diastereoisomers, we must understand the RS System of nomenclature. It is also known as the Cahn-Ingold-Prelog or CIP priority rules. Unlike D/L or (+)/(-) systems, which are based on relative structure or experimental light rotation, the RS system is based strictly on the 3D arrangement of atoms. This is the “GPS system” chemists use to name the specific 3D orientation of atoms around a chiral center.
The Core Objective
Since enantiomers and diastereoisomers have the same atoms, we need a way to distinguish between “Left-handed” and “Right-handed” versions. The designations come from Latin:
- R (Rectus): for “Right” (Clockwise).
- S (Sinister): for “Left” (Counter-clockwise).
The Sequence Rules (The CIP Rules)
The Cahn-Ingold-Prelog (CIP) priority rules are the standardized set of criteria used in organic chemistry to assign priority to groups attached to a chiral center or a double bond. This system is essential for naming molecules as R/S (for chiral centers). Step-by-Step procedure is discussed as below:
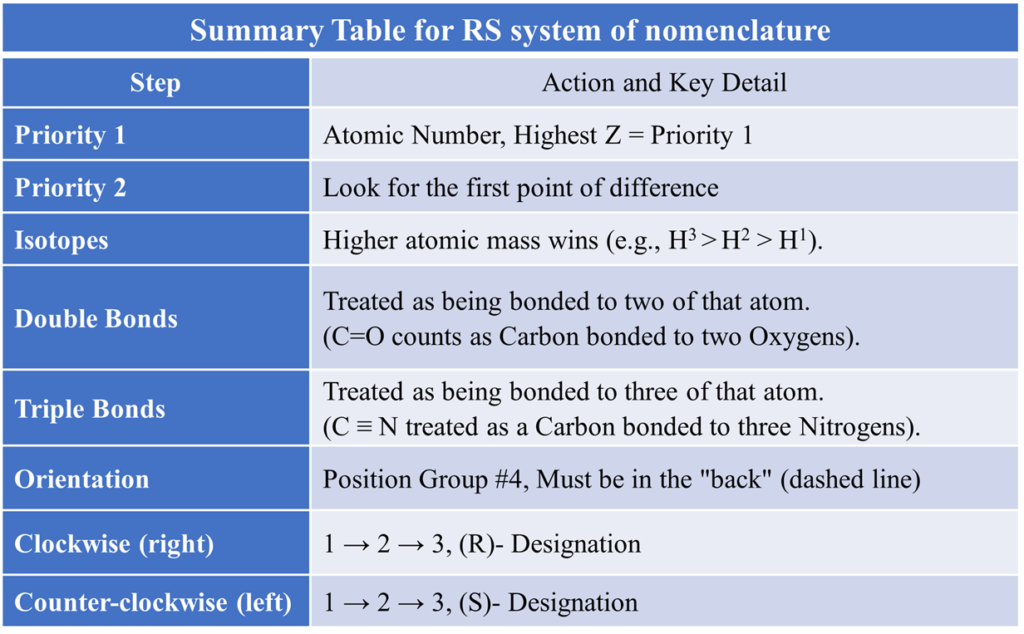
Step 1: Assign Priorities
Look at the four atoms directly attached to the chiral carbon. Based on their atomic number (Z), assign a priority number (1 for highest, 4 for lowest).
Rule 1: Atomic Number (Higher Atomic Number = Higher Priority). Look at the atoms directly attached to the chiral center. Priority 1 is assigned to the atom with the highest atomic number (Z). The lowest atomic number gets Priority 4 (usually Hydrogen)..
Example: I (53) > Br (35) > Cl (17) > F (9) > O (8) > N (7) > C (6) > H (1).
Rule 2: “Tie-Breaking”: If the atoms directly attached to the chiral carbon are the same (e.g., two Carbon atoms), look at the next atoms attached to those atoms. Create a list of atoms for each path in decreasing order of atomic number and compare them one by one. It is done until the tie is broken.
Example: An Ethyl group (-CH2CH3) has higher priority than a Methyl group (-CH3) because the Ethyl carbon is attached to (C, H, H) while the Methyl carbon is attached to (H, H, H). Since Carbon > Hydrogen, Ethyl wins.
Rule 3: “Isotopes”: If two atoms are isotopes of the same element (same atomic number), priority is given to the isotope with the higher atomic mass. Example- Hydrogen.
Order: Tritium (H3) > Deuterium H2 > Protium H1 .
Rule 4: Multiple Bonds (Double and Triple Bonds): Atoms involved in double or triple bonds are treated as if they are bonded to two or three of those atoms respectively. You duplicate the atoms. -CH=O (aldehyde) is treated as a Carbon bonded to two Oxygens. -C ≡ N (nitrile) is treated as a Carbon bonded to three Nitrogens.
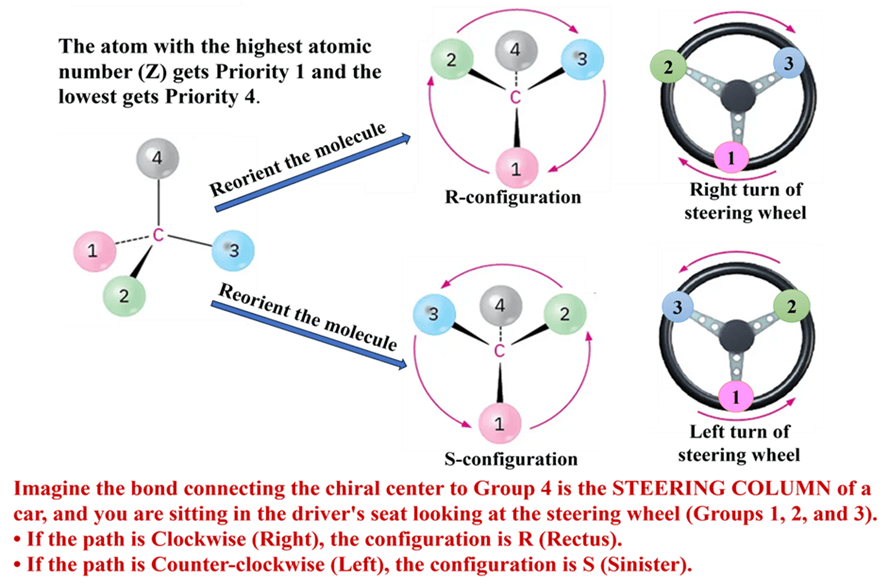
Step 2: Orient the Molecule (The “Steering Wheel” Position)
Once priorities (1, 2, 3, 4) are assigned, you must view the molecule from a specific angle. Rotate the molecule so that the group with the lowest priority (usually Hydrogen, #4) is pointing away from you ((on a dashed bond into the page).
Visualizing Trick: Imagine the bond connecting the chiral center to Group 4 is the steering column of a car, and you are sitting in the driver’s seat looking at the steering wheel (Groups 1, 2, and 3).
Step 3: Determine the Direction
Draw a curved arrow starting from Priority 1, going through Priority 2, and ending at Priority 3. Trace a path from priority 1 → 2 → 3.
- If the path is Clockwise (Right), the configuration is R (Rectus).
- If the path is Counter-clockwise (Left), the configuration is S (Sinister).
Summary Table for Examination
Examples: Alanine, Lactic Acid, Tartaric acid.
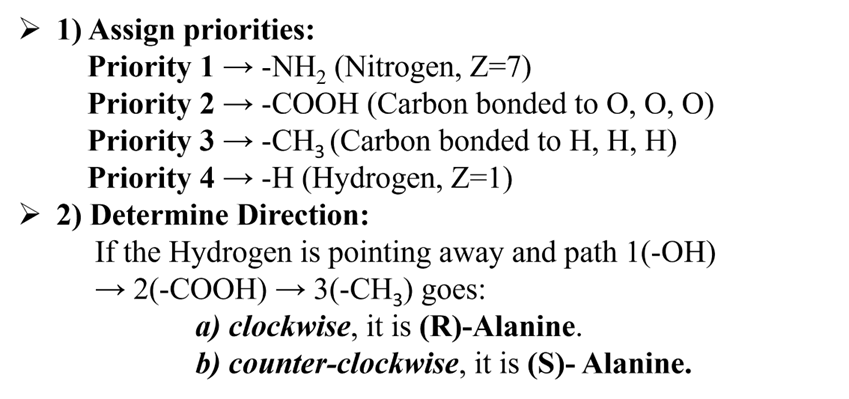
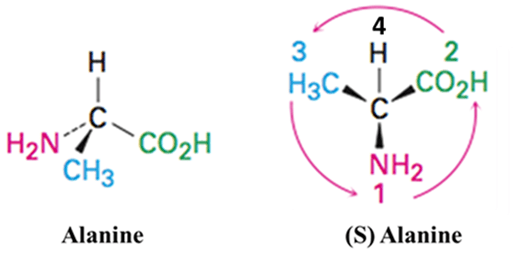
Alanine
The amino acid Alanine with a chiral center attached to different groups is priorities as below:
Lactic Acid (2-hydroxypropanoic acid)
Look at the chiral center of Lactic acid.
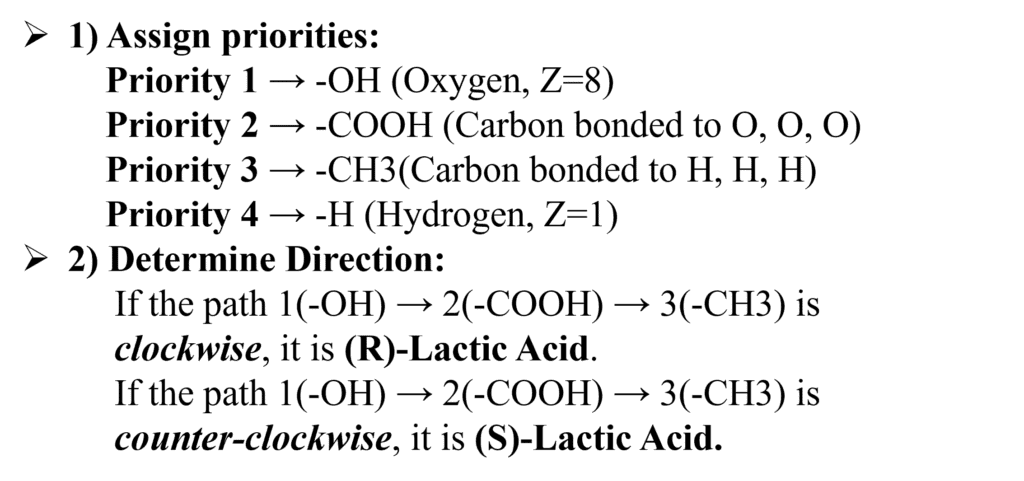
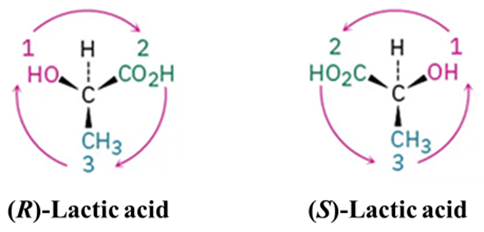
.
Tartaric acid (2,3-Dihydroxy Succinic acid)
It is a fantastic example because it has two chiral centers (Carbon-2 and Carbon-3) and requires careful application of the “tie-breaker” rule. It is also historically significant—this is the molecule Louis Pasteur used to discover chirality. Let’s focus on Carbon-2 (C2) to find its configuration. The process is identical for Carbon-3.
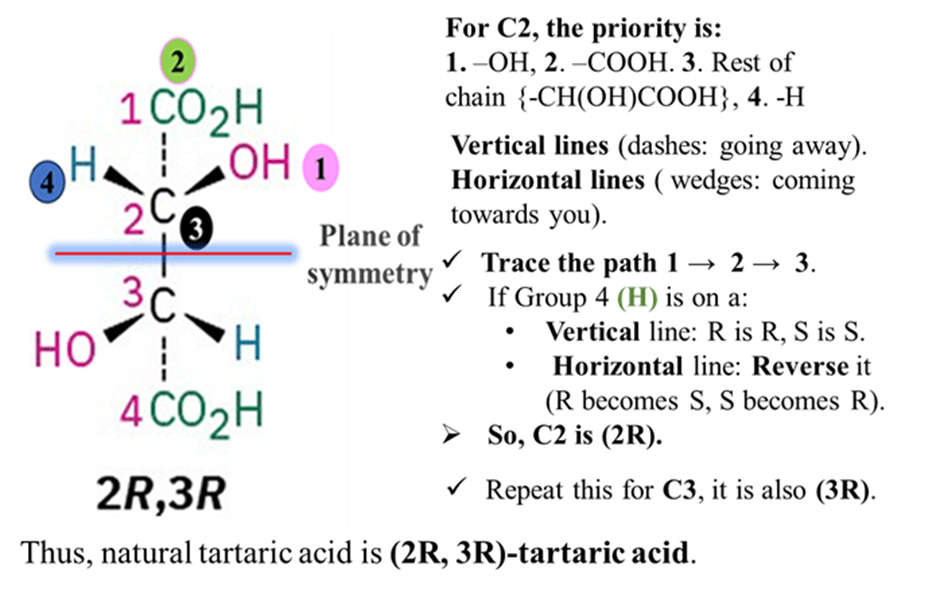
Step 1: Identify the Groups on C2
Look at Carbon-2. It is attached to four groups:
- -OH (Hydroxyl group)
- -COOH (Carboxylic acid group at the top)
- -CH(OH)COOH (The entire rest of the carbon chain at the bottom)
- -H (Hydrogen)
Step 2: Assign Priorities (The Tricky Part)
Priority 1: -OH. Oxygen (Atomic Number 8) beats Carbon (6) and Hydrogen (1).
Priority 4: -H. Hydrogen has the lowest atomic number.
Priorities 2 vs 3 (The Tie-Breaker):
Now we must compare the top group (-COOH) vs. the bottom group (-CH(OH)COOH). Both are attached via Carbon, so we look at what those carbons are attached to.
- Top Group (-COOH): The Carbon is double-bonded to one Oxygen and single-bonded to another. We treat the double bond as two bonds to Oxygen. So, list of atoms attached: (O, O, O).
- Bottom Group (-CH(OH)COOH): This Carbon (C3) is bonded to one Oxygen (from the OH), one Carbon (from the bottom COOH), and one Hydrogen. So, list of atoms attached: (O, C, H).
Comparison: Compare the lists atom by atom in descending order of atomic number:
- First atom: O vs O (Tie).
- Second atom: O vs C.
- Oxygen wins.
Therefore, the -COOH group is Priority 2, and the rest of the chain is Priority 3.
Final Ranking for C2:
- -OH
- -COOH
- Rest of chain (-CH(OH)COOH)
- -H
Step 3: Determine R or S (Using Fischer Projections)
Tartaric acid is the most commonly drawn using a Fischer Projection (flat cross shape). Here is the special rule for Fischer projections:
- Vertical lines are dashes (going away).
- Horizontal lines are wedges (coming towards you).
The Rule:
- Trace the path 1 → 2 → 3.
- If Group 4 (H) is on a Vertical line: Keep the answer (R is R, S is S).
If Group 4 (H) is on a Horizontal line: Reverse the answer (R becomes S, S becomes R).
Example Application: Natural (+)-Tartaric Acid
In naturally occurring tartaric acid:
- At C2, the -OH is on the right, and -H is on the left (Horizontal).
- Priorities: OH(1) → COOH(2) → Chain(3).
- Tracing 1 to 2 (top) to 3 (down) goes Counter-Clockwise (Left).
- This looks like S.
- BUT: The H (Group 4) is on a horizontal line (wedge).
- Reverse it: S becomes R.
So, C2 is (2R).
If you repeat this for C3, you will find it is also (3R).
Thus, natural tartaric acid is (2R, 3R)-tartaric acid.
Handling Multiple Chiral Centers
This is where it connects back to diastereoisomers. For a molecule with two chiral centers (at Carbon-2 and Carbon-3 in Tartaric acid), we name it by indicating the configuration at each:
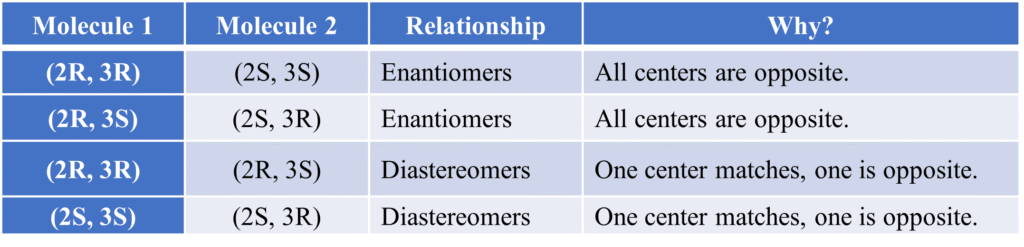
- (2R, 3R) and (2S, 3S) are Enantiomers (total mirror images).
- (2R, 3R) and (2R, 3S) are Diastereoisomers (one center matches, one is flipped).
Summary Table: Identifying Relationships via RS: This table is a “cheat sheet” for exams. If you know the RS configuration of two molecules, you can instantly tell their relationship without drawing them.
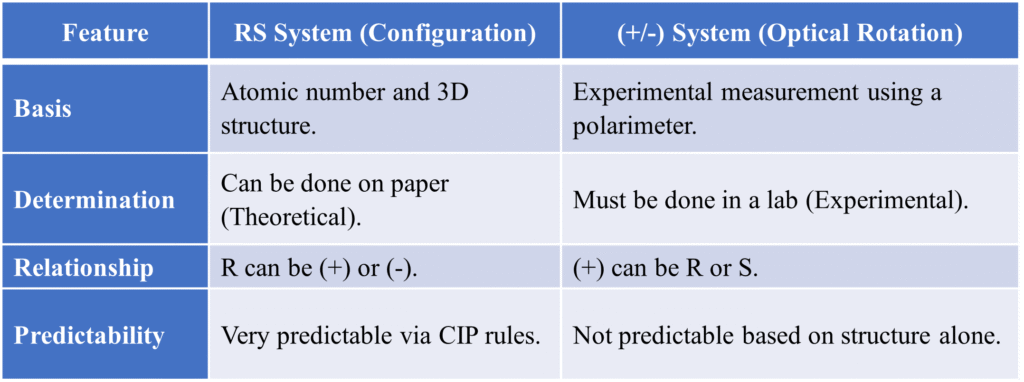
Summary Table: RS System vs. (+/-) System
It is a common mistake to think R means (+) and S means (-). This is not true.
Summary Checklist for Naming
- Identify the chiral carbon.
- Rank neighbors by atomic number.
- Put #4 in the back.
- Follow the 1-2-3 circle.
- Record as R or S.