In the context of surface coatings and chemistry, drying oils are a vital class of materials.
Definition
Drying oils are liquid vegetable or animal fats that, when exposed to air, undergo a chemical reaction (polymerization) to form a tough, solid and elastic film. Contrary to the name, they do not “dry” through the evaporation of water or solvents. Instead, they dry through auto-oxidation and cross-linking.
Chemical Composition
Drying oils are primarily triglycerides—esters of glycerol and fatty acids. Their ability to dry depends on the presence of unsaturation (double bonds) in the fatty acid chains.
- Key Fatty Acids: Linoleic, alpha-linolenic, and Oleic acids.
- The Iodine Value: It measures the degree of unsaturation.
- Drying Oils: Iodine value > 130 (e.g., Linseed oil).
- Semi-drying Oils: Iodine value 100–130 (e.g., Soybean oil).
- Non-drying Oils: Iodine value < 100 (e.g., Castor oil, Olive oil).
Mechanism of Drying (Autoxidation)
The transition from liquid to solid occurs in three main stages:
1. Induction Period: For a few hours after application, there is no visible change. During this time, natural antioxidants in the oil (like Vitamin E) are being consumed by atmospheric oxygen.
2. Oxidation (Initiation): The oil begins to absorb oxygen from the air rapidly. It attacks the C=C (double bonds) to form hydroperoxides. In linseed oil, the weight of the film can actually increase by up to 10–15% due to the added weight of oxygen molecules.
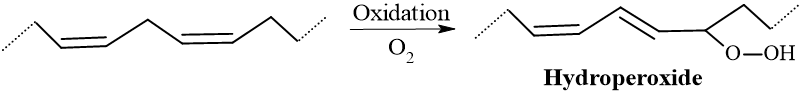
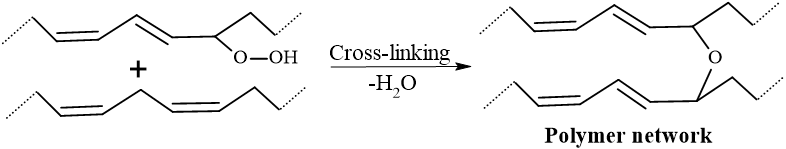
3. Polymerization (Propagation & Cross-linking): The hydroperoxides decompose into free radicals, which initiate cross-linking between the fatty acid chains. It results in a polymer network, often visible by formation of a skin-like film on samples. The viscosity increases till turning the liquid into a solid film.
The Chemical Mechanism (Step-by-Step)
This is a free-radical mechanism.
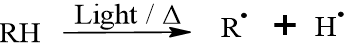
Step A: Initiation (Hydrogen Abstraction): Energy from light or heat causes a hydrogen atom to break away from a methylene group (-CH2-) located between two C=C double bonds. This creates a highly reactive carbon radical (R˙).
Step B: Oxygenation: The carbon radical reacts instantly with oxygen from the air to form a peroxy radical (ROO˙).
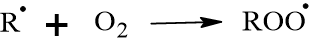
Step C: Hydroperoxide Formation:
The peroxy radical is unstable. It “steals” a hydrogen from a neighboring fatty acid chain to stabilize itself, forming a hydroperoxide (ROOH) and creating a new carbon radical. This keeps the chain reaction going.
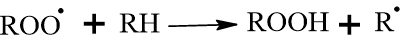
Step D: Cross-linking (The “Drying” Step): The hydroperoxides (ROOH) are unstable and decompose into alkoxy (RO˙) and hydroxyl (˙OH) radicals.
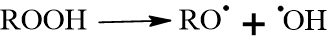
These radicals then attack the double bonds of adjacent oil molecules, creating covalent bonds between them. The new bonds such as C-C bonds, C-O-C bonds, and C-O-O-C bonds are formed. This turns individual oil molecules into a massive 3D polymer network.
The Role of Metallic Driers (Catalysts)
In a laboratory or industrial setting, natural oils dry too slowly. Metallic driers are used to speed up the decomposition of hydroperoxides.
- Primary (Surface) Driers: Cobalt (Co) and Manganese (Mn). They act at the surface to promote rapid oxygen uptake and “set-to-touch” drying.
- Secondary (Through) Driers: Lead (Pb), Zirconium (Zr), or Calcium (Ca). They promote drying throughout the entire thickness of the film, preventing the surface from “skinning” while the bottom remains liquid.
Classification with Examples
| Category | Iodine Value | Characteristics | Examples |
| Drying Oils | > 130 | Form a hard, non- sticky film rapidly. | Linseed Oil, Tung Oil |
| Semi-Drying | 100 – 130 | Form a sticky film after a long time. | Soybean Oil, Sunflower Oil |
| Non-Drying | < 100 | Do not form a solid film; remain greasy. | Olive Oil, Coconut Oil |
Factors Affecting Drying
- Number of Double Bonds: More unsaturation leads to faster drying.
- Temperature & Light: Heat and UV light accelerate the oxidation process.
- Driers (Catalysts): Metallic salts like Cobalt, Manganese, or Lead naphthenates are added to paints to speed up the drying time.
- Conjugation: Oils with conjugated double bonds (e.g., Tung oil) dry much faster than those with non-conjugated bonds (e.g., Linseed oil).
Comparison Table: Conjugated vs. Non-Conjugated
| Feature | Non-Conjugated (Linseed oil) | Conjugated (Tung/China Wood oil) |
| Structure | Double bonds separated by -CH2– | Double bonds are adjacent (-C=C-C=C-) |
| Speed | Slower (requires hydroperoxide step) | Faster (direct oxygen addition) |
| Oxygen Need | High oxygen absorption | Lower oxygen absorption |
| Film Quality | Flexible, but yellows | Harder, very water-resistant |
Applications
1. Paints and Surface Coatings
Drying oils are the most common binders (also called “vehicles”) in traditional paints. The oil holds the pigment in suspension. Once applied, it polymerizes to form a tough, weather-resistant film. Examples: Linseed oil is the standard for house paints, while Safflower or Poppy oil is used for white paints because they yellow less than linseed.
2. Varnishes and Wood Finishing
Varnishes are essentially drying oils mixed with resins. Tung Oil is used for wood finishing because it is water-resistant and provides a high-gloss, “natural” look. It is often used on boat decks and kitchen countertops.
3. Linoleum Manufacturing
Linoleum (the classic floor covering) is actually named after its primary ingredient: Linseed Oil. Linseed oil is oxidized into a thick, rubbery mass called “Linoleum cement.” This is mixed with wood flour or cork dust and pressed onto a jute backing.
4. Printing Inks
Drying oils are crucial for the printing industry, especially in offset lithography. They allow the ink to “set” quickly on paper. As the oil polymerizes, it binds the ink particles to the paper fibers
5. Putty and Sealants
Glazing putty (used to fix glass panes in window frames) is made by mixing whiting (chalk powder) with linseed oil. The oil slowly dries, creating a permanent, airtight, and waterproof seal that remains slightly flexible to accommodate the expansion of the glass.
6. Self-Healing Coatings: Recent research uses micro-encapsulated drying oils (like Tung oil) in metal coatings. When the coating is scratched, the capsules break, the oil flows out, reacts with air, and “heals” the scratch to prevent corrosion.
Summary Table
| Industry | Key Oil Used | Role of the Oil |
| Fine Arts | Poppy/Walnut Oil | Non-yellowing binder for pigments. |
| Construction | Linseed Oil | Base for putty and exterior house paints. |
| Furniture | Tung Oil | Waterproof, high-gloss wood finish. |
| Printing | Perilla/Linseed Oil | Quick-drying vehicle for inks. |
| Flooring | Linseed Oil | Primary binder for Linoleum. |