The Saponification Value (or Saponification Number) is a critical chemical constant used to characterize and assess the quality of fats and oils. It is essentially a measure of the average molecular weight (or chain length) of all the fatty acids present in a sample.
Definition and Units:
The Saponification Value is defined as the number of milligrams (mg) of potassium hydroxide (KOH) required to neutralize free fatty acids and saponify (turn into soap) the esters in one gram of fat or oil. Its unit is mg KOH / g of oil.
The Saponification Value is an index of the mean molecular weight of the fatty acids in a sample. It is inversely proportional to the average molecular weight of the fatty acids.
- High SV: Indicates the presence of short-chain fatty acids (e.g., Coconut oil, SV ≈ 250 mg KOH/g). Since the molecules are smaller and light weight, there are more molecules per gram of fat, requiring more KOH to break the ester bonds.
- Low SV: Indicates long-chain fatty acids (e.g., mustard oil, olive oil, SV ≈ 170-195 mg KOH/g). Larger and heavier molecules mean there are fewer molecules per gram, thus requiring less KOH to break the ester bonds.
Chemical Principle and reaction
Saponification is the alkaline hydrolysis of an ester. Fats and oils are triglycerides (esters of glycerol and fatty acids). When heated with an alkali (KOH), they undergo hydrolysis to form glycerol and the potassium salt of the fatty acid (soap).
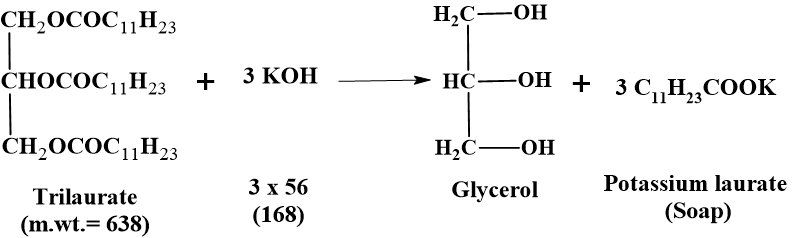
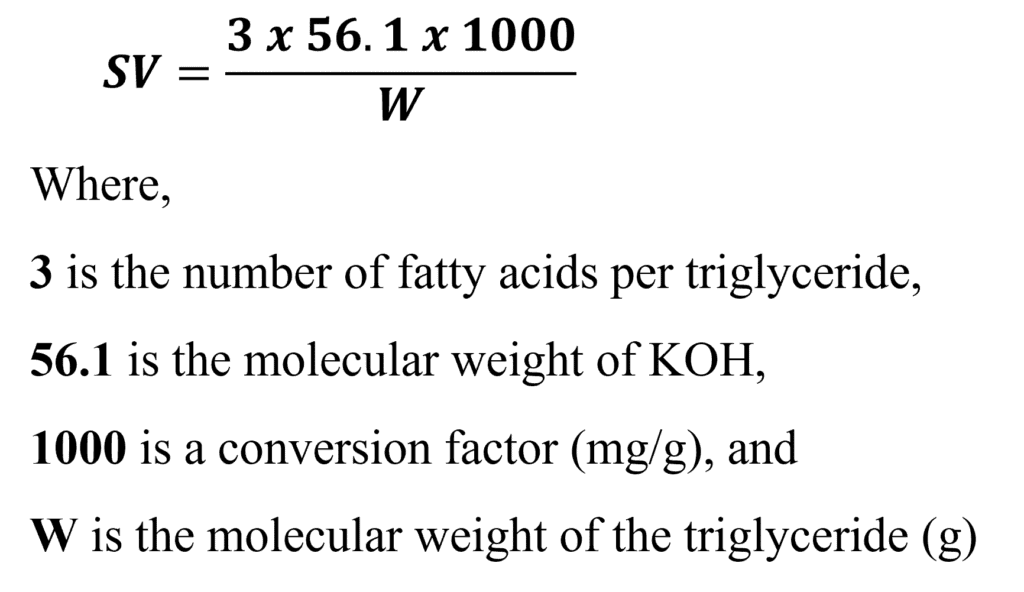
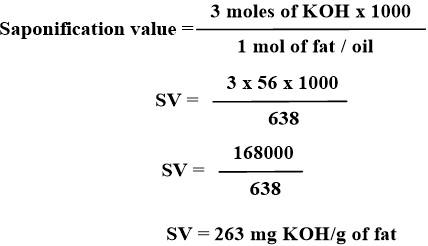
Since three (3) ester bonds are present in a triglyceride molecule, for hydrolysis three equivalents of potassium hydroxide are required to saponify one molecular weight of any fat or oil. So, the Formula for Theoretical Calculation is as below:
Experimental Procedure
Materials and Reagents
- Sample: Filtered, moisture-free oil.
- Apparatus: Quickfit / Round-bottom flask, reflux condenser, water bath, and burette.
- Alcoholic KOH (0.5N): Prepared in ethanol. Alcohol is used because fats are soluble in alcohol but not in water, allowing for a homogeneous reaction mixture.
- Standard HCl (0.5N): Used as the titrant.
- Indicator: Phenolphthalein (changes from pink to colorless).
Technical Procedure (Step-by-Step)
- Weighing: Accurately weigh approx. 2g of the oil sample into a round bottom flask.
- Addition of Alkali: Transfer exactly 25mL of 0.5N alcoholic KOH into the flask.
- Refluxing: Attach a reflux condenser and heat the mixture on a boiling water bath for 30–60 minutes. The solution should become clear, indicating complete saponification.
- Blank Run: Simultaneously, run a “Blank” experiment using 25mL of KOH without any oil. This accounts for the total concentration of KOH available.
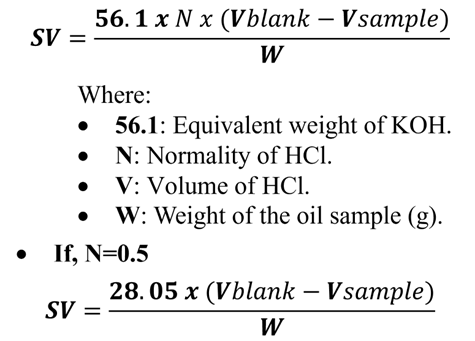
- Titration: After cooling, the excess, unreacted KOH is titrated against a standard acid (usually 0.5N HCl) using 2–3 drops of phenolphthalein indicator. Titrate both, the sample and the blank.
- V1 (Blank): High volume of HCl (no KOH consumed by oil).
- V2 (Sample): Lower volume of HCl (some KOH was used to make soap).
- Calculation Formula: The Saponification Value is calculated using:
Real-World Applications
- Soap Industry: The SV is essentially the “recipe” for soap production. It tells the manufacturer exactly how much Lye (NaOH or KOH) is needed. If the alkali is too low, the soap remains oily and greasy. If the alkali is too high, the soap becomes “harsh” or “lye-heavy,” which can burn the skin. High SV Coconut oil (short chains) produces hard, high-lathering soaps, while low SV Olive oil (long chains) produces gentler, moisturizing soaps.
- Food Industry: Used to detect adulteration. For example, if expensive butter or coconut oil (High SV) is mixed with cheaper lard or palm oil (Lower SV), the SV of the mixture will drop below the standard range. Shifting SV can indicate that the long-chain fatty acids are breaking down into smaller fragments due to oxidation or aging. It changes the chemical profile of the food.
- Purity Testing: Since mineral oils (paraffin) cannot be saponified, an unusually low SV or a cloudy solution after saponification indicates contamination with non-saponifiable matter.
- Lubricant Formulation: In engineering, SV helps determine the suitability of oils for high-temperature lubrication and their tendency to form ” a thick, semi-solid residue”.
Comparative Table:
Quick Reference
| Feature | High Saponification Value | Low Saponification Value |
| Chain Length | Short chains (e.g., C4 -C12) | Long chains (e.g., C16 -C22) |
| Molecular Weight | Low | High |
| Soap Quality | Hard, High Lather | Soft, Moisturizing |
| Example | Coconut Oil, Butterfat | Olive Oil, Rapeseed Oil |
Saponification Values of Common Oils: This table highlights how the Saponification Value (SV) changes based on the fatty acid profile of the oil.
| Oil / Fat | Saponification Value (mg KOH/g) | Primary Fatty Acids | Chain Length Type |
| Coconut Oil | 250 – 265 (High) | Lauric, Myristic | Short/Medium |
| Butter Fat (Ghee) | 220 – 230 | Butyric, Caproic | Short |
| Palm Kernel Oil | 240 – 255 | Lauric | Medium |
| Palm Oil | 190 – 205 | Palmitic, Oleic | Long |
| Olive Oil | 185 – 196 (Medium) | Oleic | Long |
| Sunflower Oil | 188 – 194 | Linoleic | Long |
| Castor Oil | 175 – 187 | Ricinoleic | Long |
| Beeswax | 80 – 105 (Low) | Wax Esters | Very Long |
Master Revision Table: Analytical Constants
| Parameter | What it Measures | Principal Reagent | Key Sample/Limit (IP) |
| Acid Value | Rancidity (Free Fatty Acids) | 0.1M KOH | Generally, < 2.0 for purity. |
| Saponification Value | Chain length / Molecular Weight | Alcoholic KOH | Coconut Oil: 250–264 (Highest) |
| Ester Value | Bound Fatty Acids | (Derived) | Beeswax: 70–80 |
| Acetyl Value | Free Hydroxyl (—OH) Groups | Acetic Anhydride | Castor Oil: more than 143 |
| Iodine Value | Double Bonds (-C=C-), Degree of Unsaturation | Wijs Reagent (ICl) | Unsaturated oils, Castor Oil: 82–90 Linseed Oil: >170 |